Selective cytotoxicity of pancratistatin-related natural Amaryllidaceae alkaloids: evaluation of the activity of two new compounds
- PMID: 17550595
- PMCID: PMC1892540
- DOI: 10.1186/1475-2867-7-10
Selective cytotoxicity of pancratistatin-related natural Amaryllidaceae alkaloids: evaluation of the activity of two new compounds
Abstract
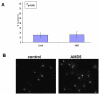
Background: Pancratistatin (PST), a compound extracted from an Amaryllidaceae (AMD) family plant, has been shown to specifically induce apoptosis in cancer cells with no/minimal toxic effect on normal cells. A systematic synthetic approach has indicated that the minimum cytotoxic pharmacophore comprises the trans-fused b/c-ring system containing the 2, 3, 4-triol unit in the C-ring. To further explore the structure-activity relationship of this group of compounds we have investigated the anti-cancer efficacy and specificity of two PST-related natural compounds, AMD4 and AMD5. Both of these compounds lack the polyhydroxylated lycorane element of PST instead having a methoxy-substituted crinane skeleton.
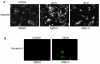
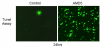
Results: Our results indicate that AMD5 has efficacy and selectivity similar to PST, albeit at a 10-fold increased concentration. Interestingly AMD4 lacks apoptotic activity.
Conclusion: Our results indicate that the phenanthridone skeleton in natural Amaryllidaceae alkaloids may be a significant common element for selectivity against cancer cells; furthermore, the configuration of the methoxy-side groups is responsible for higher binding affinity to the target protein/s thus making for a more efficient anti-cancer agent.
Figures
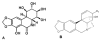
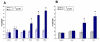
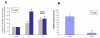
References
LinkOut - more resources
Full Text Sources

