Myocardial segment-specific model generation for simulating the electrical action of the heart
- PMID: 17550624
- PMCID: PMC1896167
- DOI: 10.1186/1475-925X-6-21
Myocardial segment-specific model generation for simulating the electrical action of the heart
Abstract
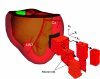
Background: Computer models of the electrical and mechanical actions of the heart, solved on geometrically realistic domains, are becoming an increasingly useful scientific tool. Construction of these models requires detailed measurement of the microstructural features which impact on the function of the heart. Currently a few generic cardiac models are in use for a wide range of simulation problems, and contributions to publicly accessible databases of cardiac structures, on which models can be solved, remain rare. This paper presents to-date the largest database of porcine left ventricular segment microstructural architecture, for use in both electrical and mechanical simulation.
Methods: Cryosectioning techniques were used to reconstruct the myofibre and myosheet orientations in tissue blocks of size ~15 x 15 x 15 mm, taken from the mid-anterior left ventricular freewall, of seven hearts. Tissue sections were gathered on orthogonal planes, and the angles of intersection of myofibres and myosheets with these planes determined automatically with a gradient intensity based algorithm. These angles were then combined to provide a description of myofibre and myosheet variation throughout the tissue, in a form able to be input to biophysically based computational models of the heart.
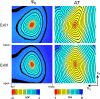
Results: Several microstructural features were common across all hearts. Myofibres rotated through 141 +/- 18 degrees (mean +/- SD) from epicardium to endocardium, in near linear fashion. In the outer two-thirds of the wall sheet angles were predominantly negative, however, in the inner one-third an abrupt change in sheet angle, with reversal in sign, was seen in six of the seven hearts. Two distinct populations of sheets with orthogonal orientations often co-existed, usually with one population dominating. The utility of the tissue structures was demonstrated by simulating the passive and active electrical responses of two of the tissue blocks to current injection. Distinct patterns of electrical response were obtained in the two tissue blocks, illustrating the importance of testing model based predictions on a variety of tissue architectures.
Conclusion: This study significantly expands the set of geometries on which models of cardiac function can be solved.
Figures

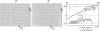


References
-
- Hunter PJ, Smaill BH, Nielsen PMF, LeGrice IJ. A mathematical model of cardiac anatomy Computational Biology of the Heart. New York: John Wiley & Sons Ltd; 1997.
-
- Nielsen P, LeGrice IJ, Smaill BH, Hunter PJ. Mathematical Model of Geometry and Fibrous Structure of the Heart. Am J Physiol. 1991;260:H1365–78. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

