Imaging protein interactions with bioluminescence resonance energy transfer (BRET) in plant and mammalian cells and tissues
- PMID: 17551013
- PMCID: PMC1891211
- DOI: 10.1073/pnas.0701987104
Imaging protein interactions with bioluminescence resonance energy transfer (BRET) in plant and mammalian cells and tissues
Abstract
FRET is a well established method for cellular and subcellular imaging of protein interactions. However, FRET obligatorily necessitates fluorescence excitation with its concomitant problems of photobleaching, autofluorescence, phototoxicity, and undesirable stimulation of photobiological processes. A sister technique, bioluminescence resonance energy transfer (BRET), avoids these problems because it uses enzyme-catalyzed luminescence; however, BRET signals usually have been too dim to image effectively in the past. Using a new generation electron bombardment-charge-coupled device camera coupled to an image splitter, we demonstrate that BRET can be used to image protein interactions in plant and animal cells and in tissues; even subcellular imaging is possible. We have applied this technology to image two different protein interactions: (i) dimerization of the developmental regulator, COP1, in plant seedlings; and (ii) CCAAT/enhancer binding protein alpha (C/EBPalpha) in the mammalian nucleus. This advance heralds a host of applications for imaging without fluorescent excitation and its consequent limitations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
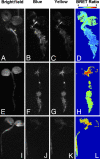
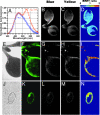
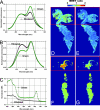
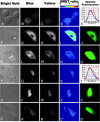
Comment in
-
Imaging protein-protein interactions in plants and single cells.Proc Natl Acad Sci U S A. 2007 Jun 12;104(24):9917-8. doi: 10.1073/pnas.0703734104. Epub 2007 Jun 5. Proc Natl Acad Sci U S A. 2007. PMID: 17551007 Free PMC article. No abstract available.
References
-
- Mendelsohn AR, Brent R. Science. 1999;284:1948–1950. - PubMed
-
- Förster T. Annalen Der Physik. 1948;2:55–75.
-
- Periasamy A, Day RN. Periasamy A, Day RN. Molecular Imaging: FRET Microscopy and Spectroscopy. New York: Oxford Univ Press; 2005. p. 321.
-
- Morin JG, Hastings JW. J Cell Physiol. 1971;77:313–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

