Metaplastic effect of apamin on LTP and paired-pulse facilitation
- PMID: 17551097
- PMCID: PMC1896089
- DOI: 10.1101/lm.571007
Metaplastic effect of apamin on LTP and paired-pulse facilitation
Abstract
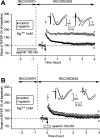
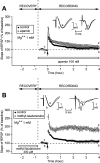
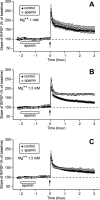
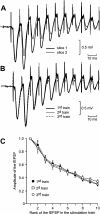
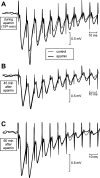
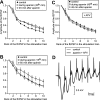
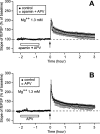
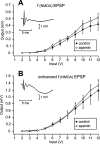
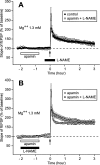
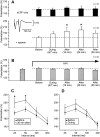
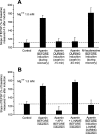
In area CA1 of hippocampal slices, a single 1-sec train of 100-Hz stimulation generally triggers a short-lasting long-term potentiation (S-LTP) of 1-2 h. Here, we found that when such a train was applied 45 min after application of the small conductance Ca(2+)-activated K(+ )(SK) channel blocker apamin, it induced a long-lasting LTP (L-LTP) of several hours, instead of an S-LTP. Apamin-induced SK channel blockage is known to resist washing. Nevertheless, the aforementioned effect is not a mere delayed effect; it is metaplastic. Indeed, when a single train was delivered to the Schaffer's collaterals during apamin application, it induced an S-LTP, like in the control situation. At the moment of this LTP induction (15th min of apamin application), the SK channel blockage was nevertheless complete. Indeed, at that time, under the influence of apamin, the amplitude of the series of field excitatory postsynaptic potentials (fEPSPs) triggered by a stimulation train was increased. We found that the metaplastic effect of apamin on LTP was crucially dependent on the NO-synthase pathway, whereas the efficacy of the NMDA receptors was not modified at the time of its occurrence. We also found that apamin produced an increase in paired-pulse facilitation not during, but after, the application of the drug. Finally, we found that the induction of each of these two metaplastic phenomena was mediated by NMDA receptors. A speculative unitary hypothesis to explain these phenomena is proposed.
Figures
References
-
- Abel T., Nguyen P.V., Barad M., Deuel T., Kandel E.R., Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. - PubMed
-
- Abraham W.C., Bear M.F. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Abraham W.C., Tate W.P. Metaplasticity: A new vista across the field of synaptic plasticity. Prog. Neurobiol. 1997;52:303–323. - PubMed
-
- Arancio O., Kiebler M., Lee C., Lev-Ram V., Tsien R., Kandel E., Hawkins R. Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell. 1996;87:1025–1035. - PubMed
-
- Behnisch T., Reymann K.G. Inhibition of apamin-sensitive calcium dependent potassium channels facilitate the induction of long-term potentiation in the CA1 region of rat hippocampus in vitro. Neurosci. Lett. 1998;253:91–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
