Asymmetry of early endosome distribution in C. elegans embryos
- PMID: 17551574
- PMCID: PMC1876258
- DOI: 10.1371/journal.pone.0000493
Asymmetry of early endosome distribution in C. elegans embryos
Abstract
Background: Endocytosis is involved in the regulation of many cellular events, including signalling, cell migration, and cell polarity. To begin to investigate roles for endocytosis in early C. elegans development, we examined the distribution and dynamics of early endosomes (EEs) in embryos.
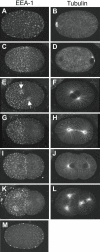
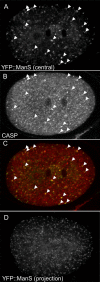
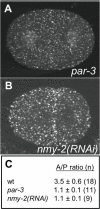
Methodology/principal findings: EEs are primarily found at the cell periphery with an initially uniform distribution after fertilization. Strikingly, we find that during the first cell cycle, EEA-1 positive EEs become enriched at the anterior cortex. In contrast, the Golgi compartment shows no asymmetry in distribution. Asymmetric enrichment of EEs depends on acto-myosin contractility and embryonic PAR polarity. In addition to their localization at the cortex, EEs are also found around the centrosome. These EEs move rapidly (1.3 microm/s) from the cortex directly to the centrosome, a speed comparable to that of the minus end directed motor dynein.
Conclusions/significance: We speculate that the asymmetry of early endosomes might play a role in cell asymmetries or fate decisions.
Conflict of interest statement
Figures


References
-
- Emery G, Knoblich JA. Endosome dynamics during development. Curr Opin Cell Biol. 2006;18:407–415. - PubMed
-
- Goldstein B, Hird SN. Specification of the anteroposterior axis in Caenorhabditis elegans. Development. 1996;122:1467–1474. - PubMed
-
- Cowan CR, Hyman AA. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature. 2004;431:92–96. - PubMed
-
- O'Connell KF, Maxwell KN, White JG. The spd-2 gene is required for polarization of the anteroposterior axis and formation of the sperm asters in the Caenorhabditis elegans zygote. Dev Biol. 2000;222:55–70. - PubMed
-
- Sadler PL, Shakes DC. Anucleate Caenorhabditis elegans sperm can crawl, fertilize oocytes and direct anterior-posterior polarization of the 1-cell embryo. Development. 2000;127:355–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

