The role of the substantia nigra pars compacta in regulating sleep patterns in rats
- PMID: 17551593
- PMCID: PMC1876809
- DOI: 10.1371/journal.pone.0000513
The role of the substantia nigra pars compacta in regulating sleep patterns in rats
Abstract
Background: As of late, dopaminergic neurotransmission has been recognized to be involved in the generation of sleep disturbances. Increasing evidence shows that sleep disturbances in Parkinson's disease (PD) patients are mostly related to the disease itself, rather than being a secondary phenomenon. Evidence contained in the literature lends support to the hypothesis that the dopaminergic nigrostriatal pathway is closely involved in the regulation of sleep patterns.
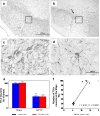
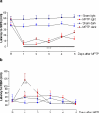
Methodology/principal findings: To test this hypothesis we examined the electrophysiological activity along the sleep-wake cycle of rats submitted to a surgically induced lesion of the SNpc by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). We demonstrated that a 50% lesion of the substantia nigra pars compacta (SNpc) suffices to produce disruptions of several parameters in the sleep-wake pattern of rats. A robust and constant decrease in the latency to the onset of slow wave sleep (SWS) was detected throughout the five days of recording in both light [F((22.16)) = 72.46, p<0.0001] and dark [F((22.16)) = 75.0, p<0.0001] periods. Also found was a pronounced increase in the percentage of sleep efficiency during the first four days of recording [F((21.15)) = 21.48, p<0.0001], in comparison to the sham group. Additionally, the reduction in the SNpc dopaminergic neurons provoked an ablation in the percentage of rapid eye movement sleep (REM) during three days of the sleep-wake recording period with a strong correlation (r = 0.91; p<0.0001) between the number of dopaminergic neurons lost and the percentage decrease of REM sleep on the first day of recording. On day 4, the percentage of REM sleep during the light and dark periods was increased, [F((22.16)) = 2.46, p<0.0007], a phenomenon consistent with REM rebound.
Conclusions/significance: We propose that dopaminergic neurons present in the SNpc possess a fundamental function in the regulation of sleep processes, particularly in promoting REM sleep.
Conflict of interest statement
Figures


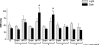


References
-
- Adler CH. Nonmotor complications in Parkinson's disease. Mov Disord. 2005;20:S23–S29. - PubMed
-
- Gagnon JF, Bedard MA, Fantini ML, Petit D, Panisset M, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology. 2002;59:585–589. - PubMed
-
- Hobson D, Lang A, Wayne MW, Razmy A, Rivest J, et al. Excessive daytime sleepiness and sudden-onset sleep in Parkinson Disease. A survey by the Canadian Movement Disorder Group. JAMA. 2002;287:455–463. - PubMed
-
- Sandyk R. Treatment with weak electromagnetic fields restores dream recall in a parkinsonian patient. Int J Neurosci. 1997;90:75–86. - PubMed
-
- Hobson JA, Pace-Schott EF, Stickgold R, Kahn D. To dream or not to dream? Relevant data from new neuroimaging and electrophysiological studies. Curr Opin Neurobiol. 1998;8:239–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

