Inhibition of nitric oxide and soluble guanylyl cyclase signaling affects olfactory neuron activity in the moth, Manduca sexta
- PMID: 17551736
- PMCID: PMC2629079
- DOI: 10.1007/s00359-007-0227-9
Inhibition of nitric oxide and soluble guanylyl cyclase signaling affects olfactory neuron activity in the moth, Manduca sexta
Abstract
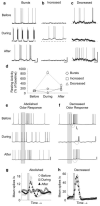
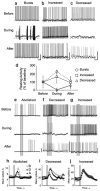
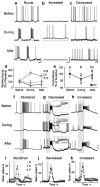
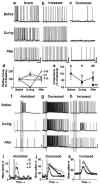
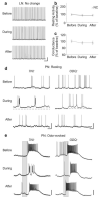
Nitric oxide is emerging as an important modulator of many physiological processes including olfaction, yet the function of this gas in the processing of olfactory information remains poorly understood. In the antennal lobe of the moth, Manduca sexta, nitric oxide is produced in response to odor stimulation, and many interneurons express soluble guanylyl cyclase, a well-characterized nitric oxide target. We used intracellular recording and staining coupled with pharmacological manipulation of nitric oxide and soluble guanylyl cyclase to test the hypothesis that nitric oxide modulates odor responsiveness in olfactory interneurons through soluble guanylyl cyclase-dependent pathways. Nitric oxide synthase inhibition resulted in pronounced effects on the resting level of firing and the responses to odor stimulation in most interneurons. Effects ranged from bursting to strong attenuation of activity and were often accompanied by membrane depolarization coupled with a change in input resistance. Blocking nitric oxide activation of soluble guanylyl cyclase signaling mimicked the effects of nitric oxide synthase inhibitors in a subset of olfactory neurons, while other cells were differentially affected by this treatment. Together, these results suggest that nitric oxide is required for proper olfactory function, and likely acts through soluble guanylyl cyclase-dependent and -independent mechanisms in different subsets of neurons.
Figures

Similar articles
-
Odorant-evoked nitric oxide signals in the antennal lobe of Manduca sexta.J Neurosci. 2004 Jul 7;24(27):6070-7. doi: 10.1523/JNEUROSCI.0710-04.2004. J Neurosci. 2004. PMID: 15240798 Free PMC article.
-
Nitric oxide-soluble guanylyl cyclase signaling regulates corticostriatal transmission and short-term synaptic plasticity of striatal projection neurons recorded in vivo.Neuropharmacology. 2010 Mar;58(3):624-31. doi: 10.1016/j.neuropharm.2009.11.011. Epub 2009 Dec 5. Neuropharmacology. 2010. PMID: 19969007 Free PMC article.
-
Expression of nitric oxide synthase and soluble guanylyl cyclase in the developing olfactory system of Manduca sexta.J Comp Neurol. 2000 Jun 26;422(2):191-205. doi: 10.1002/(sici)1096-9861(20000626)422:2<191::aid-cne4>3.0.co;2-c. J Comp Neurol. 2000. PMID: 10842227
-
Regulation of nitric oxide and soluble guanylyl cyclase.Brain Res Bull. 2004 Feb 15;62(6):505-15. doi: 10.1016/S0361-9230(03)00102-3. Brain Res Bull. 2004. PMID: 15036565 Review.
-
[Nitric oxide. Potentiation of NO-dependent activation of soluble guanylate cyclase--(patho)physiological and pharmacotherapeutical significance].Biomed Khim. 2007 Jul-Aug;53(4):385-99. Biomed Khim. 2007. PMID: 18035720 Review. Russian.
Cited by
-
Construction and sequence sampling of deep-coverage, large-insert BAC libraries for three model lepidopteran species.BMC Genomics. 2009 Jun 26;10:283. doi: 10.1186/1471-2164-10-283. BMC Genomics. 2009. PMID: 19558662 Free PMC article.
-
A review of the actions of Nitric Oxide in development and neuronal function in major invertebrate model systems.AIMS Neurosci. 2019 Aug 19;6(3):146-174. doi: 10.3934/Neuroscience.2019.3.146. eCollection 2019. AIMS Neurosci. 2019. PMID: 32341974 Free PMC article. Review.
-
The role of nitric oxide in memory is modulated by diurnal time.Front Syst Neurosci. 2014 Apr 11;8:59. doi: 10.3389/fnsys.2014.00059. eCollection 2014. Front Syst Neurosci. 2014. PMID: 24847218 Free PMC article.
-
Mitral and tufted cells are potential cellular targets of nitration in the olfactory bulb of aged mice.PLoS One. 2013;8(3):e59673. doi: 10.1371/journal.pone.0059673. Epub 2013 Mar 18. PLoS One. 2013. PMID: 23527248 Free PMC article.
-
NOS1 mutations cause hypogonadotropic hypogonadism with sensory and cognitive deficits that can be reversed in infantile mice.Sci Transl Med. 2022 Oct 5;14(665):eabh2369. doi: 10.1126/scitranslmed.abh2369. Epub 2022 Oct 5. Sci Transl Med. 2022. PMID: 36197968 Free PMC article.
References
-
- Ahern G, Klyachko V, Jackson M. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. TINS. 2002;25:510–517. doi:10.1016/S0166-2236(02)02254-3. - PubMed
-
- Bicker G, Schmachtenberg O, De Vente J. The nitric oxide/cyclic GMP messenger system in olfactory pathways of the locust brain. Eur J Neurosci. 1996;8:2635–2643. doi:10.1111/j.1460-9568.1996.tb01558.x. - PubMed
-
- Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi:10.1016/0896-6273(91)90374-9. - PubMed
-
- Breer H, Shepherd GM. Implications of the NO/cGMP system for olfaction. Trends Neurosci. 1993;16:5–9. doi:10.1016/0166-2236(93)90040-S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

