Damaging fatigue loading stimulates increases in periosteal vascularity at sites of bone formation in the rat ulna
- PMID: 17551770
- PMCID: PMC3680514
- DOI: 10.1007/s00223-007-9031-3
Damaging fatigue loading stimulates increases in periosteal vascularity at sites of bone formation in the rat ulna
Abstract
Bone formation in a variety of contexts depends on angiogenesis; however, there are few reports of the vascular response to osteogenic skeletal loading. We used the rat forelimb compression model to characterize vascular changes after fatigue loading. The right forelimbs of 72 adult rats were loaded cyclically in vivo to one of four displacement levels, to produce four discrete levels of ulnar damage. Rats were killed 3-14 days after loading, and their vasculature was perfused with silicone rubber. Transverse histological sections were cut along the ulnar diaphysis. We quantified vessel number, average vessel area, total vessel area, and bone area. On day 3, we observed a dramatic periosteal expansion near the ulnar midshaft, with significant increases in periosteal vascularity; total vessel area was increased 250-450% (P < 0.001). Vascularity remained elevated on days 7 and 14. Vessel number and average vessel area were not correlated (P = 0.09) and contributed independently to total vascular increases. Bone area was not increased on day 3 but on days 7 and 14 was increased significantly in all displacement groups (P < 0.01) due to periosteal woven bone formation. Vascular and bone changes depended on longitudinal location (P < 0.001), with peak increases 2 mm distal to the midshaft. Vascular and bone changes also depended on displacement level (P < 0.005), with greater increases at higher levels of fatigue displacement. We conclude that skeletal fatigue loading induces a rapid increase in periosteal vascularity, followed by an increase in bone area. The angiogenic-osteogenic response is spatially coordinated and scaled to the level of the mechanical stimulus.
Figures

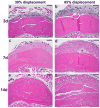


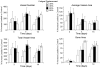
Similar articles
-
In vivo static creep loading of the rat forelimb reduces ulnar structural properties at time-zero and induces damage-dependent woven bone formation.Bone. 2008 May;42(5):942-9. doi: 10.1016/j.bone.2008.01.004. Epub 2008 Jan 26. Bone. 2008. PMID: 18295561 Free PMC article.
-
Stress fracture healing: fatigue loading of the rat ulna induces upregulation in expression of osteogenic and angiogenic genes that mimic the intramembranous portion of fracture repair.Bone. 2009 Feb;44(2):320-30. doi: 10.1016/j.bone.2008.09.010. Epub 2008 Oct 7. Bone. 2009. PMID: 18950737 Free PMC article.
-
In vivo skeletal imaging of 18F-fluoride with positron emission tomography reveals damage- and time-dependent responses to fatigue loading in the rat ulna.Bone. 2006 Aug;39(2):229-36. doi: 10.1016/j.bone.2006.01.149. Epub 2006 Mar 13. Bone. 2006. PMID: 16533624
-
Multiscale computational and experimental approaches to elucidate bone and ligament mechanobiology using the ulna-radius-interosseous membrane construct as a model system.Technol Health Care. 2012;20(5):363-78. doi: 10.3233/THC-2012-0686. Technol Health Care. 2012. PMID: 23079942 Review.
-
H Vessel Formation as a Marker for Enhanced Bone Healing in Irradiated Distraction Osteogenesis.Semin Plast Surg. 2024 Jan 19;38(1):31-38. doi: 10.1055/s-0043-1778039. eCollection 2024 Feb. Semin Plast Surg. 2024. PMID: 38495069 Free PMC article. Review.
Cited by
-
Antagonizing the αv β3 integrin inhibits angiogenesis and impairs woven but not lamellar bone formation induced by mechanical loading.J Bone Miner Res. 2014 Sep;29(9):1970-80. doi: 10.1002/jbmr.2223. J Bone Miner Res. 2014. PMID: 24644077 Free PMC article.
-
Nitric oxide-mediated vasodilation increases blood flow during the early stages of stress fracture healing.J Appl Physiol (1985). 2014 Feb 15;116(4):416-24. doi: 10.1152/japplphysiol.00957.2013. Epub 2013 Dec 19. J Appl Physiol (1985). 2014. PMID: 24356518 Free PMC article.
-
Histopathological features of bone regeneration in a canine segmental ulnar defect model.Diagn Pathol. 2014 Mar 17;9:59. doi: 10.1186/1746-1596-9-59. Diagn Pathol. 2014. Retraction in: Diagn Pathol. 2016 Nov 2;11(1):124. doi: 10.1186/s13000-016-0573-4. PMID: 24636669 Free PMC article. Retracted.
-
Skeletal vascular perfusion is altered in chronic kidney disease.Bone Rep. 2018 May 4;8:215-220. doi: 10.1016/j.bonr.2018.05.001. eCollection 2018 Jun. Bone Rep. 2018. PMID: 29955640 Free PMC article.
-
HIF-1α change in serum and callus during fracture healing in ovariectomized mice.Int J Clin Exp Pathol. 2015 Jan 1;8(1):117-26. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 25755698 Free PMC article.
References
-
- Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. J Bone Miner Res. 2006;21:183–192. - PubMed
-
- Pechak DG, Kujawa MJ, Caplan AI. Morphological and histochemical events during first bone formation in embryonic chick limbs. Bone. 1986;7:441–458. - PubMed
-
- Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. American Journal of Physiology - Cell Physiology. 2001;280:C1358–1366. - PubMed
-
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature Medicine. 1999;5:623–628. - PubMed
-
- Glowacki J. Angiogenesis in fracture repair. Clin Orthop Rel Res. 1998;355S:S82–S89. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

