In vitro cell culture infectivity assay for human noroviruses
- PMID: 17552092
- PMCID: PMC2725917
- DOI: 10.3201/eid1303.060549
In vitro cell culture infectivity assay for human noroviruses
Abstract
Human noroviruses cause severe, self-limiting gastroenteritis that typically lasts 24-48 hours. Because of the lack of suitable tissue culture or animal models, the true nature of norovirus pathogenesis remains unknown. We show, for the first time, that noroviruses can infect and replicate in a physiologically relevant 3-dimensional (3-D), organoid model of human small intestinal epithelium. This level of cellular differentiation was achieved by growing the cells on porous collagen-I coated microcarrier beads under conditions of physiological fluid shear in rotating wall vessel bioreactors. Microscopy, PCR, and fluorescent in situ hybridization provided evidence of norovirus infection. Cytopathic effect and norovirus RNA were detected at each of the 5 cell passages for genogroup I and II viruses. Our results demonstrate that the highly differentiated 3-D cell culture model can support the natural growth of human noroviruses, whereas previous attempts that used differentiated monolayer cultures failed.
Figures
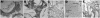
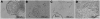
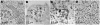


Comment in
-
Cell culture assay for human noroviruses.Emerg Infect Dis. 2007 Jul;13(7):1117; author reply 1117-8. doi: 10.3201/eid1307.070131. Emerg Infect Dis. 2007. PMID: 18214197 Free PMC article. No abstract available.
References
-
- Parashar U, Quiroz ES, Mounts AW, Monroe SS, Fankhauser RL, Ando T, et al. “Norwalk-like viruses.” Public health consequences and outbreak management. MMWR Recomm Rep. 2001;50(RR-9):1–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
