Diversity and distribution of Borrelia hermsii
- PMID: 17552097
- PMCID: PMC2725891
- DOI: 10.3201/eid1303.060958
Diversity and distribution of Borrelia hermsii
Abstract
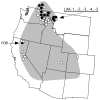
Borrelia hermsii is the most common cause of tickborne relapsing fever in North America. DNA sequences of the 16S-23S rDNA noncoding intergenic spacer (IGS) region were determined for 37 isolates of this spirochete. These sequences distinguished the 2 genomic groups of B. hermsii identified previously with other loci. Multiple IGS genotypes were identified among isolates from an island, which suggested that birds might play a role in dispersing these spirochetes in nature. In support of this theory, all stages of the tick vector Ornithodoros hermsi fed successfully on birds in the laboratory and advanced in their life cycle. B. hermsii produced a detectable spirochetemia in 1 chicken inoculated subcutaneously. Additional work is warranted to explore the role of birds as enzootic hosts for this relapsing fever spirochete.
Figures
References
-
- Davis GE. Species unity or plurality of the relapsing fever spirochetes. In: Moulton FR, editor. A symposium on relapsing fever in the Americas. Washington: American Association for the Advancement of Science;1942. p. 41–7.
-
- Schwan TG, Raffel SJ, Schrumpf ME, Policastro PF, Rawlings JA, Lane RS, et al. Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for tick-borne relasping fever in Florida. J Clin Microbiol. 2005;43:3851–9. 10.1128/JCM.43.8.3851-3859.2005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
