Effectiveness of neuraminidase inhibitors for preventing staff absenteeism during pandemic influenza
- PMID: 17552099
- PMCID: PMC2725890
- DOI: 10.3201/eid1303.060309
Effectiveness of neuraminidase inhibitors for preventing staff absenteeism during pandemic influenza
Abstract
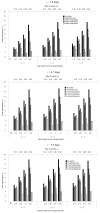
We used a deterministic SEIR (susceptible-exposed-infectious-removed) meta-population model, together with scenario, sensitivity, and simulation analyses, to determine stockpiling strategies for neuraminidase inhibitors that would minimize absenteeism among healthcare workers. A pandemic with a basic reproductive number (R0) of 2.5 resulted in peak absenteeism of 10%. Treatment decreased peak absenteeism to 8%, while 8 weeks' prophylaxis reduced it to 2%. For pandemics with higher R0, peak absenteeism exceeded 20% occasionally and 6 weeks' prophylaxis reduced peak absenteeism by 75%. Insufficient duration of prophylaxis increased peak absenteeism compared with treatment only. Earlier pandemic detection and initiation of prophylaxis may render shorter prophylaxis durations ineffective. Eight weeks' prophylaxis substantially reduced peak absenteeism under a broad range of assumptions for severe pandemics (peak absenteeism > 10%). Small investments in treatment and prophylaxis, if adequate and timely, can reduce absenteeism among essential staff.
Figures
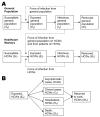


 .
†Tx refers to treatment; Rx refers to prophylaxis.
.
†Tx refers to treatment; Rx refers to prophylaxis.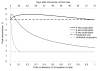
References
-
- World Health Organization. Avian influenza and human pandemic influenza: summary report. Meeting held in Geneva, Switzerland, 7–9 Nov 2005. [cited 2006 Jan 15]. Available from http://www.who.int/mediacentre/events/2005/avian_influenza/summary_repor...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
