Functional respiratory morphology in the newborn quokka wallaby (Setonix brachyurus)
- PMID: 17553103
- PMCID: PMC2375791
- DOI: 10.1111/j.1469-7580.2007.00744.x
Functional respiratory morphology in the newborn quokka wallaby (Setonix brachyurus)
Abstract
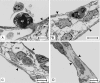
A morphological and morphometric study of the lung of the newborn quokka wallaby (Setonix brachyurus) was undertaken to assess its morphofunctional status at birth. Additionally, skin structure and morphometry were investigated to assess the possibility of cutaneous gas exchange. The lung was at canalicular stage and comprised a few conducting airways and a parenchyma of thick-walled tubules lined by stretches of cuboidal pneumocytes alternating with squamous epithelium, with occasional portions of thin blood-gas barrier. The tubules were separated by abundant intertubular mesenchyme, aggregations of developing capillaries and mesenchymal cells. Conversion of the cuboidal pneumocytes to type I cells occurred through cell broadening and lamellar body extrusion. Superfluous cuboidal cells were lost through apoptosis and subsequent clearance by alveolar macrophages. The establishment of the thin blood-gas barrier was established through apposition of the incipient capillaries to the formative thin squamous epithelium. The absolute volume of the lung was 0.02 +/- 0.001 cm(3) with an air space surface area of 4.85 +/- 0.43 cm(2). Differentiated type I pneumocytes covered 78% of the tubular surface, the rest 22% going to long stretches of type II cells, their precursors or low cuboidal transitory cells with sparse lamellar bodies. The body weight-related diffusion capacity was 2.52 +/- 0.56 mL O(2) min(-1) kg(-1). The epidermis was poorly developed, and measured 29.97 +/- 4.88 microm in thickness, 13% of which was taken by a thin layer of stratum corneum, measuring 4.87 +/- 0.98 microm thick. Superficial capillaries were closely associated with the epidermis, showing the possibility that the skin also participated in some gaseous exchange. Qualitatively, the neonate quokka lung had the basic constituents for gas exchange but was quantitatively inadequate, implying the significance of percutaneous gas exchange.
Figures



Similar articles
-
Morphological analysis of the postnatally developing marsupial lung: The quokka wallaby.Anat Rec. 2001 Mar 1;262(3):253-65. doi: 10.1002/1097-0185(20010301)262:3<253::AID-AR1025>3.0.CO;2-B. Anat Rec. 2001. PMID: 11241194
-
Morphology and morphometry of the normal lung of the adult vervet monkey (Cercopithecus aethiops).Am J Anat. 1988 Nov;183(3):258-67. doi: 10.1002/aja.1001830308. Am J Anat. 1988. PMID: 3213831
-
Morphometry and allometry of the postnatal lung development in the quokka wallaby (Setonix brachyurus): a light microscopic study.Respir Physiol Neurobiol. 2003 Feb 19;134(1):43-55. doi: 10.1016/s1569-9048(02)00204-5. Respir Physiol Neurobiol. 2003. PMID: 12573880
-
Fetal and postnatal development of the lung.Annu Rev Physiol. 1984;46:617-28. doi: 10.1146/annurev.ph.46.030184.003153. Annu Rev Physiol. 1984. PMID: 6370120 Review.
-
Development, structure, and function of a novel respiratory organ, the lung-air sac system of birds: to go where no other vertebrate has gone.Biol Rev Camb Philos Soc. 2006 Nov;81(4):545-79. doi: 10.1017/S1464793106007111. Epub 2006 Oct 12. Biol Rev Camb Philos Soc. 2006. PMID: 17038201 Review.
Cited by
-
Comparative anatomy of neonates of the three major mammalian groups (monotremes, marsupials, placentals) and implications for the ancestral mammalian neonate morphotype.J Anat. 2017 Dec;231(6):798-822. doi: 10.1111/joa.12689. Epub 2017 Sep 28. J Anat. 2017. PMID: 28960296 Free PMC article.
-
Development of the terminal air spaces in the gray short-tailed opossum (Monodelphis domestica)- 3D reconstruction by microcomputed tomography.PLoS One. 2024 Feb 16;19(2):e0292482. doi: 10.1371/journal.pone.0292482. eCollection 2024. PLoS One. 2024. PMID: 38363783 Free PMC article.
-
Development of the skin in the eastern quoll (Dasyurus viverrinus) with focus on cutaneous gas exchange in the early postnatal period.J Anat. 2021 Feb;238(2):426-445. doi: 10.1111/joa.13316. Epub 2020 Sep 25. J Anat. 2021. PMID: 32974934 Free PMC article.
-
Skin structure in newborn marsupials with focus on cutaneous gas exchange.J Anat. 2018 Jun 26;233(3):311-27. doi: 10.1111/joa.12843. Online ahead of print. J Anat. 2018. PMID: 29947022 Free PMC article.
-
Vascular Niche in Lung Alveolar Development, Homeostasis, and Regeneration.Front Bioeng Biotechnol. 2019 Nov 12;7:318. doi: 10.3389/fbioe.2019.00318. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31781555 Free PMC article. Review.
References
-
- Baudinette RV, Ganon BJ, Ryall RG, Frapell PB. Changes in metabolic rates and blood respiratory characteristics during pouch development of a marsupial, Macropus eugenii. Respir Physiol. 1988;72:219–228. - PubMed
-
- Burri PH, Weibel ER. Ultrastructure and morphometry of the developing lung. In: Hudson WA, editor. Development of the Lung. New York: Marcel Dekker; 1977. pp. 215–268.
-
- Burri PH. Lung development and pulmonary angiogenesis. In: Gaultier C, Bourbon JR, Post M, editors. Lung Development. Oxford: Oxford University Press; 1999. pp. 122–151.
-
- Burri PH, Dbaly J, Weibel ER. The postnatal growth of the rat lung. I. Morphometry. Anat Rec. 1974;178:711–730. - PubMed
-
- Burri PH, Haenni B, Tschanz SA, Makanya AN. Morphometry and allometry of the postnatal marsupial lung development, an ultrastructural study. Respir Physiol Neurobiol. 2003;138:309–324. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

