Resistance of a vaccinia virus A34R deletion mutant to spontaneous rupture of the outer membrane of progeny virions on the surface of infected cells
- PMID: 17553539
- PMCID: PMC2048979
- DOI: 10.1016/j.virol.2007.05.015
Resistance of a vaccinia virus A34R deletion mutant to spontaneous rupture of the outer membrane of progeny virions on the surface of infected cells
Abstract
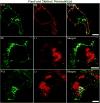
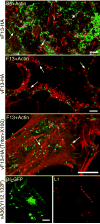
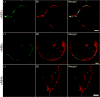
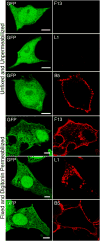
The extracellular form of vaccinia virus is referred to as an enveloped virion (EV) because it contains an additional lipoprotein membrane surrounding the infectious mature virion (MV) that must be discarded prior to cell fusion and entry. Most EVs adhere to the surface of the parent cell and mediate spread of the infection to adjacent cells. Here we show that some attached EVs have ruptured envelopes. Rupture was detected by fluorescence microscopy of unfixed and unpermeabilized cells using antibodies to the F13 and L1 proteins, which line the inner side of the EV membrane and the outer side of the MV membrane, respectively. The presence of ruptured EV membranes was confirmed by immunogold transmission electron microscopy. EVs with broken membranes were present on several cell lines examined including one deficient in glycosaminoglycans, which are thought to play a role in breakage of the EV membrane prior to fusion of the MV. No correlation was found between EVs with ruptured membranes and actin tail formation. Studies with several mutant viruses indicated that EV membranes lacking the A34 protein were unbroken. This result was consistent with other properties of A34R deletion mutants including resistance of the EV membrane to polyanions, small plaque formation and low infectivity that can be increased by disruption of the EV membrane by freezing and thawing.
Figures


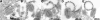
Similar articles
-
Vaccinia Virus Phospholipase Protein F13 Promotes Rapid Entry of Extracellular Virions into Cells.J Virol. 2018 May 14;92(11):e02154-17. doi: 10.1128/JVI.02154-17. Print 2018 Jun 1. J Virol. 2018. PMID: 29540596 Free PMC article.
-
The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus.J Virol. 1997 May;71(5):3904-15. doi: 10.1128/JVI.71.5.3904-3915.1997. J Virol. 1997. PMID: 9094667 Free PMC article.
-
Vaccinia virus glycoprotein A34R is required for infectivity of extracellular enveloped virus.J Virol. 1996 Jan;70(1):272-81. doi: 10.1128/JVI.70.1.272-281.1996. J Virol. 1996. PMID: 8523536 Free PMC article.
-
The exit of vaccinia virus from infected cells.Virus Res. 2004 Dec;106(2):189-97. doi: 10.1016/j.virusres.2004.08.015. Virus Res. 2004. PMID: 15567497 Review.
-
Poxvirus cell entry: how many proteins does it take?Viruses. 2012 May;4(5):688-707. doi: 10.3390/v4050688. Epub 2012 Apr 27. Viruses. 2012. PMID: 22754644 Free PMC article. Review.
Cited by
-
Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture.EMBO J. 2011 Jul 26;30(17):3647-61. doi: 10.1038/emboj.2011.245. EMBO J. 2011. PMID: 21792173 Free PMC article.
-
A Deleted Deletion Site in a New Vector Strain and Exceptional Genomic Stability of Plaque-Purified Modified Vaccinia Ankara (MVA).Virol Sin. 2020 Apr;35(2):212-226. doi: 10.1007/s12250-019-00176-3. Epub 2019 Dec 12. Virol Sin. 2020. PMID: 31833037 Free PMC article.
-
Acidic residues in the membrane-proximal stalk region of vaccinia virus protein B5 are required for glycosaminoglycan-mediated disruption of the extracellular enveloped virus outer membrane.J Gen Virol. 2009 Jul;90(Pt 7):1582-1591. doi: 10.1099/vir.0.009092-0. Epub 2009 Mar 4. J Gen Virol. 2009. PMID: 19264647 Free PMC article.
-
Expression of the A56 and K2 proteins is sufficient to inhibit vaccinia virus entry and cell fusion.J Virol. 2009 Feb;83(4):1546-54. doi: 10.1128/JVI.01684-08. Epub 2008 Nov 26. J Virol. 2009. PMID: 19036815 Free PMC article.
-
Vaccinia virus A56/K2 fusion regulatory protein interacts with the A16 and G9 subunits of the entry fusion complex.J Virol. 2008 Jun;82(11):5153-60. doi: 10.1128/JVI.00162-08. Epub 2008 Mar 19. J Virol. 2008. PMID: 18353946 Free PMC article.
References
-
- Carter GC, Law M, Hollinshead M, Smith GL. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J Gen Virol. 2005;86:1279–1290. - PubMed
-
- Condit RC, Moussatche N, Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv Virus Res. 2006;66:31–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

