Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective
- PMID: 17553668
- PMCID: PMC2233890
- DOI: 10.1016/j.cellsig.2007.04.012
Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective
Abstract
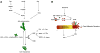
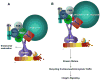
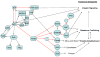
Cell migration is critical for many physiological processes and is often misregulated in developmental disorders and pathological conditions including cancer and neurodegeneration. MAPK signaling and the Rho family of proteins are known regulators of cell migration that exert their influence on cellular cytoskeleton during cell adhesion and migration. Here we review data supporting the view that localized ERK signaling mediated through recently identified scaffold proteins may regulate cell migration.
Figures

Similar articles
-
Cell migration: Rho GTPases lead the way.Dev Biol. 2004 Jan 1;265(1):23-32. doi: 10.1016/j.ydbio.2003.06.003. Dev Biol. 2004. PMID: 14697350 Review.
-
Advances in Rho-dependent actin regulation and oncogenic transformation.Curr Opin Genet Dev. 2002 Feb;12(1):36-43. doi: 10.1016/s0959-437x(01)00261-1. Curr Opin Genet Dev. 2002. PMID: 11790552 Review.
-
Novel mechanism for gonadotropin-releasing hormone neuronal migration involving Gas6/Ark signaling to p38 mitogen-activated protein kinase.Mol Cell Biol. 2002 Jan;22(2):599-613. doi: 10.1128/MCB.22.2.599-613.2002. Mol Cell Biol. 2002. PMID: 11756555 Free PMC article.
-
The role of p42/44 MAPK and protein kinase B in connective tissue growth factor induced extracellular matrix protein production, cell migration, and actin cytoskeletal rearrangement in human mesangial cells.J Biol Chem. 2002 Nov 15;277(46):44187-94. doi: 10.1074/jbc.M203715200. Epub 2002 Sep 5. J Biol Chem. 2002. PMID: 12218048
-
Regulation of actin-based cell migration by cAMP/PKA.Biochim Biophys Acta. 2004 Jul 5;1692(2-3):159-74. doi: 10.1016/j.bbamcr.2004.03.005. Biochim Biophys Acta. 2004. PMID: 15246685 Review.
Cited by
-
Pref-1 interacts with fibronectin to inhibit adipocyte differentiation.Mol Cell Biol. 2010 Jul;30(14):3480-92. doi: 10.1128/MCB.00057-10. Epub 2010 May 10. Mol Cell Biol. 2010. PMID: 20457810 Free PMC article.
-
The Mechanobiology of the Actin Cytoskeleton in Stem Cells during Differentiation and Interaction with Biomaterials.Stem Cells Int. 2018 Oct 8;2018:2891957. doi: 10.1155/2018/2891957. eCollection 2018. Stem Cells Int. 2018. PMID: 30402108 Free PMC article. Review.
-
Shoc2-tranduced ERK1/2 motility signals--Novel insights from functional genomics.Cell Signal. 2016 May;28(5):448-459. doi: 10.1016/j.cellsig.2016.02.005. Epub 2016 Feb 11. Cell Signal. 2016. PMID: 26876614 Free PMC article.
-
Spatial control of Shoc2-scaffold-mediated ERK1/2 signaling requires remodeling activity of the ATPase PSMC5.J Cell Sci. 2015 Dec 1;128(23):4428-41. doi: 10.1242/jcs.177543. Epub 2015 Oct 30. J Cell Sci. 2015. PMID: 26519477 Free PMC article.
-
The homolog of the five SH3-domain protein (HOFI/SH3PXD2B) regulates lamellipodia formation and cell spreading.PLoS One. 2011;6(8):e23653. doi: 10.1371/journal.pone.0023653. Epub 2011 Aug 23. PLoS One. 2011. PMID: 21886807 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

