X-rays induce distinct patterns of somatic mutation in fetal versus adult hematopoietic cells
- PMID: 17553756
- PMCID: PMC2063444
- DOI: 10.1016/j.dnarep.2007.04.005
X-rays induce distinct patterns of somatic mutation in fetal versus adult hematopoietic cells
Abstract
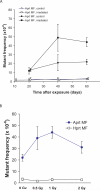
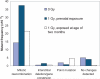
There are a variety of mechanisms and pathways whereby cells safeguard their genomes in the face of environmental insults that damage DNA. Whether each of these pathways is equally robust at specific developmental stages in mammals and whether they are also modulated in a tissue-specific manner, however, are unclear. Here, we report that ionizing radiation (IR) produces different types of somatic mutations in fetal cells compared with adult cells of the same lineage. While 1 Gy of X-ray significantly induced intragenic point mutations in T cells of adult mice, no point mutational effect was observed when applied to fetuses. Fetal exposure to IR, on the other hand, led to a significant elevation of mitotic recombination in T cells, which was not observed in adults. Base excision repair (BER) activity was significantly lower in fetal hematopoietic cells than in adult cells, due to a low level of DNA polymerase beta, the rate-limiting enzyme in BER. In fetal hematopoietic cells, this low BER activity, together with a high rate of proliferation, causes X-ray-induced DNA lesions, such as base damage, single strand breaks and double strand breaks, to be repaired by homologous recombination, which we observe as mitotic recombination. Higher BER activity and a relatively lower rate of cell proliferation likely contribute to the significant induction of DNA point mutations in adults. Thus, the mutational response to IR is at least partly determined by the availability of specific repair pathways and other developmentally regulated phenotypes, such as mitotic index.
Figures

Similar articles
-
Induction and repair of DNA strand breaks and 1-beta-D-arabinofuranosylcytosine-detectable sites in 40-75 kVp X-irradiated compared to 60Co gamma-irradiated human cell lines.Radiat Res. 1988 Apr;114(1):168-85. Radiat Res. 1988. PMID: 3353503
-
Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double-strand breaks in DNA.Int J Radiat Biol Relat Stud Phys Chem Med. 1988 Jan;53(1):39-47. doi: 10.1080/09553008814550401. Int J Radiat Biol Relat Stud Phys Chem Med. 1988. PMID: 3257477
-
Base excision repair intermediates induce p53-independent cytotoxic and genotoxic responses.J Biol Chem. 2003 Oct 10;278(41):39951-9. doi: 10.1074/jbc.M306592200. Epub 2003 Jul 25. J Biol Chem. 2003. PMID: 12882965
-
Base Excision Repair in the Immune System: Small DNA Lesions With Big Consequences.Front Immunol. 2020 May 29;11:1084. doi: 10.3389/fimmu.2020.01084. eCollection 2020. Front Immunol. 2020. PMID: 32547565 Free PMC article. Review.
-
Ionizing radiation and genetic risks XIV. Potential research directions in the post-genome era based on knowledge of repair of radiation-induced DNA double-strand breaks in mammalian somatic cells and the origin of deletions associated with human genomic disorders.Mutat Res. 2005 Oct 15;578(1-2):333-70. doi: 10.1016/j.mrfmmm.2005.06.020. Epub 2005 Aug 5. Mutat Res. 2005. PMID: 16084534 Review.
Cited by
-
Ionizing radiation is a potent inducer of mitotic recombination in mouse embryonic stem cells.Mutat Res. 2011 Oct 1;715(1-2):1-6. doi: 10.1016/j.mrfmmm.2011.06.017. Epub 2011 Jul 23. Mutat Res. 2011. PMID: 21802432 Free PMC article.
-
Mismatch and base excision repair proficiency in murine embryonic stem cells.DNA Repair (Amst). 2011 Apr 3;10(4):445-51. doi: 10.1016/j.dnarep.2011.01.008. Epub 2011 Feb 18. DNA Repair (Amst). 2011. PMID: 21315663 Free PMC article.
-
Involvement of DNA polymerase β overexpression in the malignant transformation induced by benzo[a]pyrene.Toxicology. 2013 Jul 5;309:73-80. doi: 10.1016/j.tox.2013.04.017. Epub 2013 May 4. Toxicology. 2013. PMID: 23652152 Free PMC article.
-
Evaluating biomarkers to model cancer risk post cosmic ray exposure.Life Sci Space Res (Amst). 2016 Jun;9:19-47. doi: 10.1016/j.lssr.2016.05.004. Epub 2016 May 21. Life Sci Space Res (Amst). 2016. PMID: 27345199 Free PMC article. Review.
-
The bright and the dark sides of DNA repair in stem cells.J Biomed Biotechnol. 2010;2010:845396. doi: 10.1155/2010/845396. Epub 2010 Apr 8. J Biomed Biotechnol. 2010. PMID: 20396397 Free PMC article. Review.
References
-
- ICRP Biological Effects after Prenatal Irradiation (Embryo and Fetus). ICRP Publication. Ann ICRP. 2003;90 - PubMed
-
- Sasaki S, Kasuga T. Life shortening and carcinogenesis in mice irradiated at perinatal period with gamma rays. In: Thompson RC, Mahaffey JA, editors. Lifespan Radiation Effects Studies in Animals: What Can They Tell Us? National Technical Information Service; Springfield, USA: 1986. pp. 357–367.
-
- Liang L, Deng L, Shao C, Stambrook PJ, Tischfield JA. vivo loss of heterozygosity in T-cells of B6C3F1 Aprt(+/-) mice. Environ.Mol.Mutagen. 2000;35:150–157. - PubMed
-
- Shao C, Yin M, Deng L, Stambrook PJ, Doetschman T, Tischfield JA. Loss of heterozygosity and point mutation at Aprt locus in T cells and fibroblasts of Pms2-/-mice. Oncogene. 2002;21:2840–2845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

