The effect of temperature and thermal acclimation on the sustainable performance of swimming scup
- PMID: 17553779
- PMCID: PMC2442851
- DOI: 10.1098/rstb.2007.2083
The effect of temperature and thermal acclimation on the sustainable performance of swimming scup
Abstract
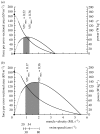
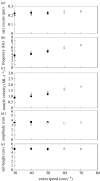
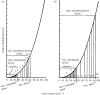
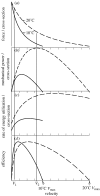
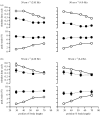
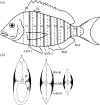
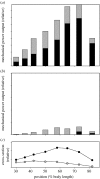
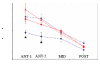
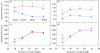
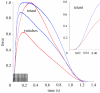
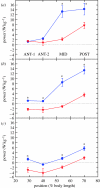
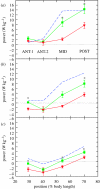
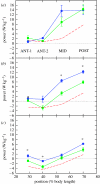
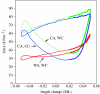
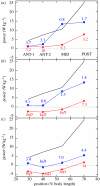
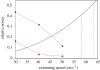
There is a significant reduction in overall maximum power output of muscle at low temperatures due to reduced steady-state (i.e. maximum activation) power-generating capabilities of muscle. However, during cyclical locomotion, a further reduction in power is due to the interplay between non-steady-state contractile properties of muscle (i.e. rates of activation and relaxation) and the stimulation and the length-change pattern muscle undergoes in vivo. In particular, even though the relaxation rate of scup red muscle is slowed greatly at cold temperatures (10 degrees C), warm-acclimated scup swim with the same stimulus duty cycles at cold as they do at warm temperature, not affording slow-relaxing muscle any additional time to relax. Hence, at 10 degrees C, red muscle generates extremely low or negative work in most parts of the body, at all but the slowest swimming speeds. Do scup shorten their stimulation duration and increase muscle relaxation rate during cold acclimation? At 10 degrees C, electromyography (EMG) duty cycles were 18% shorter in cold-acclimated scup than in warm-acclimated scup. But contrary to the expectations, the red muscle did not have a faster relaxation rate, rather, cold-acclimated muscle had an approximately 50% faster activation rate. By driving cold- and warm-acclimated muscle through cold- and warm-acclimated conditions, we found a very large increase in red muscle power during swimming at 10 degrees C. As expected, reducing stimulation duration markedly increased power output. However, the increased rate of activation alone produced an even greater effect. Hence, to fully understand thermal acclimation, it is necessary to examine the whole system under realistic physiological conditions.
Figures





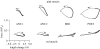

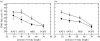
Similar articles
-
The influence of thermal acclimation on power production during swimming. I. In vivo stimulation and length change pattern of scup red muscle.J Exp Biol. 2001 Feb;204(Pt 3):409-18. doi: 10.1242/jeb.204.3.409. J Exp Biol. 2001. PMID: 11171294
-
The influence of thermal acclimation on power production during swimming. II. Mechanics of scup red muscle under in vivo conditions.J Exp Biol. 2001 Feb;204(Pt 3):419-30. doi: 10.1242/jeb.204.3.419. J Exp Biol. 2001. PMID: 11171295
-
Thermal acclimation to cold alters myosin content and contractile properties of rainbow smelt, Osmerus mordax, red muscle.Comp Biochem Physiol A Mol Integr Physiol. 2016 Jun;196:46-53. doi: 10.1016/j.cbpa.2016.02.021. Epub 2016 Mar 3. Comp Biochem Physiol A Mol Integr Physiol. 2016. PMID: 26945595
-
Influence of temperature on muscle recruitment and muscle function in vivo.Am J Physiol. 1990 Aug;259(2 Pt 2):R210-22. doi: 10.1152/ajpregu.1990.259.2.R210. Am J Physiol. 1990. PMID: 2201215 Review.
-
Temperature acclimation and metabolism in ectotherms with particular reference to teleost fish.Symp Soc Exp Biol. 1987;41:67-93. Symp Soc Exp Biol. 1987. PMID: 3332497 Review.
Cited by
-
Critical swimming speed at different temperatures for small-bodied freshwater native riverine fish species.Sci Rep. 2024 Aug 9;14(1):18526. doi: 10.1038/s41598-024-69355-x. Sci Rep. 2024. PMID: 39122770 Free PMC article.
-
Key factors explaining critical swimming speed in freshwater fish: a review and statistical analysis for Iberian species.Sci Rep. 2020 Nov 3;10(1):18947. doi: 10.1038/s41598-020-75974-x. Sci Rep. 2020. PMID: 33144649 Free PMC article. Review.
-
Rising floor and dropping ceiling: organ heterogeneity in response to cold acclimation of the largest extant amphibian.Proc Biol Sci. 2022 Oct 12;289(1984):20221394. doi: 10.1098/rspb.2022.1394. Epub 2022 Oct 5. Proc Biol Sci. 2022. PMID: 36196548 Free PMC article.
-
The effects of warm thermal variability on metabolism and swimming performance in wild Atlantic salmon (Salmo salar).J Fish Biol. 2025 Mar;106(3):893-907. doi: 10.1111/jfb.15996. Epub 2024 Nov 24. J Fish Biol. 2025. PMID: 39581221 Free PMC article.
-
Physiological mechanisms linking cold acclimation and the poleward distribution limit of a range-extending marine fish.Conserv Physiol. 2020 May 26;8(1):coaa045. doi: 10.1093/conphys/coaa045. eCollection 2020. Conserv Physiol. 2020. PMID: 32494362 Free PMC article.
References
-
- Altringham J.D, Johnston I.A. Modelling muscle power output in a swimming fish. J. Exp. Biol. 1990;148:395–402.
-
- Altringham J.D, Wardle C.S, Smith C.I. Myotomal muscle function at different locations in the body of a swimming fish. J. Exp. Biol. 1993;182:191–206.
-
- Anderson E.J, McGillis W.R, Grosenbaugh M.A. The boundary layer of swimming fish. J. Exp. Biol. 2001;204:81–102. - PubMed
-
- Beddow T.A, van Leeuwen J.L, Johnston I.A. Swimming kinematics of fast starts are altered by temperature acclimation in the marine fish Myoxocephalus scorpius. J. Exp. Biol. 1995;198:203–208. - PubMed
-
- Bennett A.F. The thermal dependence of locomotor capacity. Am. J. Physiol. 1990;259:R253–R258. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

