Effects of nucleic acid local structure and magnesium ions on minus-strand transfer mediated by the nucleic acid chaperone activity of HIV-1 nucleocapsid protein
- PMID: 17553835
- PMCID: PMC1919501
- DOI: 10.1093/nar/gkm375
Effects of nucleic acid local structure and magnesium ions on minus-strand transfer mediated by the nucleic acid chaperone activity of HIV-1 nucleocapsid protein
Abstract
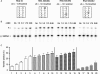
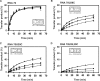
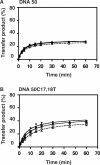
HIV-1 nucleocapsid protein (NC) is a nucleic acid chaperone, which is required for highly specific and efficient reverse transcription. Here, we demonstrate that local structure of acceptor RNA at a potential nucleation site, rather than overall thermodynamic stability, is a critical determinant for the minus-strand transfer step (annealing of acceptor RNA to (-) strong-stop DNA followed by reverse transcriptase (RT)-catalyzed DNA extension). In our system, destabilization of a stem-loop structure at the 5' end of the transactivation response element (TAR) in a 70-nt RNA acceptor (RNA 70) appears to be the major nucleation pathway. Using a mutational approach, we show that when the acceptor has a weak local structure, NC has little or no effect. In this case, the efficiencies of both annealing and strand transfer reactions are similar. However, when NC is required to destabilize local structure in acceptor RNA, the efficiency of annealing is significantly higher than that of strand transfer. Consistent with this result, we find that Mg2+ (required for RT activity) inhibits NC-catalyzed annealing. This suggests that Mg2+ competes with NC for binding to the nucleic acid substrates. Collectively, our findings provide new insights into the mechanism of NC-dependent and -independent minus-strand transfer.
Figures



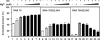
Similar articles
-
Specific interactions between HIV-1 nucleocapsid protein and the TAR element.J Mol Biol. 2005 May 20;348(5):1059-77. doi: 10.1016/j.jmb.2005.03.046. Epub 2005 Apr 7. J Mol Biol. 2005. PMID: 15854644
-
During the early phase of HIV-1 DNA synthesis, nucleocapsid protein directs hybridization of the TAR complementary sequences via the ends of their double-stranded stem.J Mol Biol. 2006 Mar 10;356(5):1180-92. doi: 10.1016/j.jmb.2005.12.038. Epub 2005 Dec 27. J Mol Biol. 2006. PMID: 16406407
-
Nucleic acid conformational changes essential for HIV-1 nucleocapsid protein-mediated inhibition of self-priming in minus-strand transfer.J Mol Biol. 2003 Jan 3;325(1):1-10. doi: 10.1016/s0022-2836(02)01177-4. J Mol Biol. 2003. PMID: 12473448
-
The chaperoning and assistance roles of the HIV-1 nucleocapsid protein in proviral DNA synthesis and maintenance.Int J Biochem Cell Biol. 2004 Sep;36(9):1668-86. doi: 10.1016/j.biocel.2004.02.024. Int J Biochem Cell Biol. 2004. PMID: 15183337 Review.
-
Initiation of HIV-1 reverse transcription and functional role of nucleocapsid-mediated tRNA/viral genome interactions.Virus Res. 2012 Nov;169(2):324-39. doi: 10.1016/j.virusres.2012.06.006. Epub 2012 Jun 18. Virus Res. 2012. PMID: 22721779 Review.
Cited by
-
The nonstructural protein 2C of a Picorna-like virus displays nucleic acid helix destabilizing activity that can be functionally separated from its ATPase activity.J Virol. 2013 May;87(9):5205-18. doi: 10.1128/JVI.00245-13. Epub 2013 Feb 28. J Virol. 2013. PMID: 23449794 Free PMC article.
-
Effect of Mg(2+) and Na(+) on the nucleic acid chaperone activity of HIV-1 nucleocapsid protein: implications for reverse transcription.J Mol Biol. 2009 Feb 27;386(3):773-88. doi: 10.1016/j.jmb.2008.12.073. Epub 2009 Jan 6. J Mol Biol. 2009. PMID: 19154740 Free PMC article.
-
Blocking premature reverse transcription fails to rescue the HIV-1 nucleocapsid-mutant replication defect.Retrovirology. 2011 Jun 17;8:46. doi: 10.1186/1742-4690-8-46. Retrovirology. 2011. PMID: 21682883 Free PMC article.
-
Fundamental differences between the nucleic acid chaperone activities of HIV-1 nucleocapsid protein and Gag or Gag-derived proteins: biological implications.Virology. 2010 Sep 30;405(2):556-67. doi: 10.1016/j.virol.2010.06.042. Epub 2010 Jul 23. Virology. 2010. PMID: 20655566 Free PMC article.
-
The N-terminal zinc finger and flanking basic domains represent the minimal region of the human immunodeficiency virus type-1 nucleocapsid protein for targeting chaperone function.Biochemistry. 2013 Nov 19;52(46):8226-36. doi: 10.1021/bi401250a. Epub 2013 Nov 6. Biochemistry. 2013. PMID: 24144434 Free PMC article.
References
-
- Darlix J-L, Lapadat-Tapolsky M, de Rocquigny H, Roques BP. First glimpses at structure-function relationships of the nucleocapsid protein of retroviruses. J. Mol. Biol. 1995;254:523–537. - PubMed
-
- Rein A, Henderson LE, Levin JG. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends Biochem. Sci. 1998;23:297–301. - PubMed
-
- Levin JG, Guo J, Rouzina I, Musier-Forsyth K. Nucleic acid chaperone activity of HIV-1 nucleocapsid protein: critical role in reverse transcription and molecular mechanism. Prog. Nucleic Acid Res. Mol. Biol. 2005;80:217–286. - PubMed
-
- De Guzman RN, Wu ZR, Stalling CC, Pappalardo L, Borer PN, Summers MF. Structure of the HIV-1 nucleocapsid protein bound to the SL3 ψ-RNA recognition element. Science. 1998;279:384–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

