Caveolar endocytosis is critical for BK virus infection of human renal proximal tubular epithelial cells
- PMID: 17553887
- PMCID: PMC1951339
- DOI: 10.1128/JVI.00924-07
Caveolar endocytosis is critical for BK virus infection of human renal proximal tubular epithelial cells
Abstract
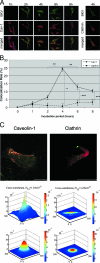
In recent years, BK virus (BKV) nephritis after renal transplantation has become a severe problem. The exact mechanisms of BKV cell entry and subsequent intracellular trafficking remain unknown. Since human renal proximal tubular epithelial cells (HRPTEC) represent a main natural target of BKV nephritis, analysis of BKV infection of HRPTEC is necessary to obtain additional insights into BKV biology and to develop novel strategies for the treatment of BKV nephritis. We coincubated HRPTEC with BKV and the cholesterol-depleting agents methyl beta cyclodextrin (MBCD) and nystatin (Nys), drugs inhibiting caveolar endocytosis. The percentage of infected cells (detected by immunofluorescence) and the cellular levels of BKV large T antigen expression (detected by Western blot analysis) were significantly decreased in both MBCD- and Nys-treated HPRTEC compared to the level in HRPTEC incubated with BKV alone. HRPTEC infection by BKV was also tested after small interfering RNA (siRNA)-dependent depletion of either the caveolar structural protein caveolin-1 (Cav-1) or clathrin, the major structural protein of clathrin-coated pits. BKV infection was inhibited in HRPTEC transfected with Cav-1 siRNA but not in HRPTEC transfected with clathrin siRNA. The colocalization of labeled BKV particles with either Cav-1 or clathrin was investigated by using fluorescent microscopy and image cross-correlation spectroscopy. The rate of colocalization of BKV with Cav-1 peaked at 4 h after incubation. Colocalization with clathrin was insignificant at all time points. These results suggest that BKV entered into HRPTEC via caveolae, not clathrin-coated pits, and that BKV is maximally associated with caveolae at 4 h after infection, prior to relocation to a different intracellular compartment.
Figures

Similar articles
-
Repression of BK virus infection of human renal proximal tubular epithelial cells by pravastatin.Transplantation. 2008 May 15;85(9):1311-7. doi: 10.1097/TP.0b013e31816c4ec5. Transplantation. 2008. PMID: 18475189 Free PMC article.
-
Intracellular trafficking pathway of BK Virus in human renal proximal tubular epithelial cells.Virology. 2008 Feb 20;371(2):336-49. doi: 10.1016/j.virol.2007.09.030. Epub 2007 Oct 31. Virology. 2008. PMID: 17976677 Free PMC article.
-
Caveolae may enable albumin to enter human renal glomerular endothelial cells.J Cell Biochem. 2015 Jun;116(6):1060-9. doi: 10.1002/jcb.25061. J Cell Biochem. 2015. PMID: 25640083
-
Mechanisms of BK virus infection of renal cells and therapeutic implications.J Clin Virol. 2015 Oct;71:59-62. doi: 10.1016/j.jcv.2015.08.003. Epub 2015 Aug 6. J Clin Virol. 2015. PMID: 26295751 Free PMC article. Review.
-
Update on BK virus entry and intracellular trafficking.Transpl Infect Dis. 2006 Jun;8(2):62-7. doi: 10.1111/j.1399-3062.2006.00153.x. Transpl Infect Dis. 2006. PMID: 16734628 Review.
Cited by
-
Sending mixed signals: polyomavirus entry and trafficking.Curr Opin Virol. 2021 Apr;47:95-105. doi: 10.1016/j.coviro.2021.02.004. Epub 2021 Mar 6. Curr Opin Virol. 2021. PMID: 33690104 Free PMC article. Review.
-
Identification of potassium and calcium channel inhibitors as modulators of polyomavirus endosomal trafficking.Antiviral Res. 2020 Jul;179:104819. doi: 10.1016/j.antiviral.2020.104819. Epub 2020 May 8. Antiviral Res. 2020. PMID: 32389733 Free PMC article.
-
Polyoma virus nephropathy in kidney transplantation.World J Transplant. 2017 Dec 24;7(6):329-338. doi: 10.5500/wjt.v7.i6.329. World J Transplant. 2017. PMID: 29312862 Free PMC article. Review.
-
BK virus nephritis after renal transplantation.Clin J Am Soc Nephrol. 2008 Mar;3 Suppl 2(Suppl 2):S68-75. doi: 10.2215/CJN.02770707. Clin J Am Soc Nephrol. 2008. PMID: 18309005 Free PMC article. Review.
-
Diabetic Nephropathy and Gaseous Modulators.Antioxidants (Basel). 2023 May 12;12(5):1088. doi: 10.3390/antiox12051088. Antioxidants (Basel). 2023. PMID: 37237955 Free PMC article. Review.
References
-
- Allen, M. J., and N. Rushton. 1994. Use of the CytoTox 96 assay in routine biocompatibility testing in vitro. Promega Notes 45:7-10.
-
- Celik, B., and P. S. Randhawa. 2004. Glomerular change in BK virus nephropathy. Hum. Pathol. 35:367-370. - PubMed
-
- Chen, Y., and L. C. Norkin. 1999. Extracellular simian virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp. Cell Res. 246:83-90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

