Reversible inhibition of the fusion activity of measles virus F protein by an engineered intersubunit disulfide bridge
- PMID: 17553889
- PMCID: PMC1951371
- DOI: 10.1128/JVI.00754-07
Reversible inhibition of the fusion activity of measles virus F protein by an engineered intersubunit disulfide bridge
Abstract
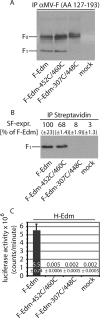
In search of target sites for the development of paramyxovirus inhibitors, we have engineered disulfide bridges to introduce covalent links into the prefusion F protein trimer of measles virus. F-Edm-452C/460C, predicted to bridge head and stalk domains of different F monomers, shows a high degree of proteolytic maturation and surface expression, predominantly as stable, dithiothreitol-sensitive trimers, but no fusion activity. Reduction of disulfide bridges partially restores activity. These findings underscore the importance of reversible intersubunit interactions between the stalk and head domains for F activity. Noncovalent small molecules mimicking this behavior may constitute a potent strategy for preventing paramyxovirus entry.
Figures


Similar articles
-
Membrane fusion triggering: three modules with different structure and function in the upper half of the measles virus attachment protein stalk.J Biol Chem. 2012 Nov 9;287(46):38543-51. doi: 10.1074/jbc.M112.410563. Epub 2012 Sep 24. J Biol Chem. 2012. PMID: 23007387 Free PMC article.
-
Mutations in the Fusion Protein of Measles Virus That Confer Resistance to the Membrane Fusion Inhibitors Carbobenzoxy-d-Phe-l-Phe-Gly and 4-Nitro-2-Phenylacetyl Amino-Benzamide.J Virol. 2017 Nov 14;91(23):e01026-17. doi: 10.1128/JVI.01026-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28904193 Free PMC article.
-
Primary resistance mechanism of the canine distemper virus fusion protein against a small-molecule membrane fusion inhibitor.Virus Res. 2019 Jan 2;259:28-37. doi: 10.1016/j.virusres.2018.10.003. Epub 2018 Oct 5. Virus Res. 2019. PMID: 30296457
-
Measles Virus Fusion Protein: Structure, Function and Inhibition.Viruses. 2016 Apr 21;8(4):112. doi: 10.3390/v8040112. Viruses. 2016. PMID: 27110811 Free PMC article. Review.
-
Structure and function of a paramyxovirus fusion protein.Biochim Biophys Acta. 2003 Jul 11;1614(1):73-84. doi: 10.1016/s0005-2736(03)00164-0. Biochim Biophys Acta. 2003. PMID: 12873767 Review.
Cited by
-
Design and Structure of an Engineered Disulfide-Stabilized Influenza Virus Hemagglutinin Trimer.J Virol. 2015 Jul;89(14):7417-20. doi: 10.1128/JVI.00808-15. Epub 2015 Apr 29. J Virol. 2015. PMID: 25926650 Free PMC article.
-
Measles Resurgence and Drug Development.Curr Opin Virol. 2020 Apr;41:8-17. doi: 10.1016/j.coviro.2020.02.007. Epub 2020 Apr 1. Curr Opin Virol. 2020. PMID: 32247280 Free PMC article. Review.
-
Structure-based antigen design: a strategy for next generation vaccines.Trends Biotechnol. 2008 Dec;26(12):659-67. doi: 10.1016/j.tibtech.2008.08.002. Epub 2008 Oct 30. Trends Biotechnol. 2008. PMID: 18977045 Free PMC article. Review.
-
Structure and stabilization of the Hendra virus F glycoprotein in its prefusion form.Proc Natl Acad Sci U S A. 2016 Jan 26;113(4):1056-61. doi: 10.1073/pnas.1523303113. Epub 2015 Dec 28. Proc Natl Acad Sci U S A. 2016. PMID: 26712026 Free PMC article.
-
Stabilisation of Viral Membrane Fusion Proteins in Prefusion Conformation by Structure-Based Design for Structure Determination and Vaccine Development.Viruses. 2022 Aug 18;14(8):1816. doi: 10.3390/v14081816. Viruses. 2022. PMID: 36016438 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention. 2005. Progress in reducing measles mortality—worldwide, 1999-2003. Morb. Mortal. Wkly. Rep. 54:200-203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

