Temporal activity patterns in thermosensory neurons of freely moving Caenorhabditis elegans encode spatial thermal gradients
- PMID: 17553981
- PMCID: PMC6672141
- DOI: 10.1523/JNEUROSCI.1032-07.2007
Temporal activity patterns in thermosensory neurons of freely moving Caenorhabditis elegans encode spatial thermal gradients
Abstract
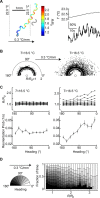
Our understanding of the operation of neurons and neuronal circuits has come primarily from probing their activity in dissected, anesthetized, or restrained animals. However, the behaviorally relevant operation of neurons and neuronal circuits occurs within intact animals as they freely perform behavioral tasks. The small size and transparency of the nematode Caenorhabditis elegans make it an ideal system for noninvasive, optical measurements of neuronal activity. Here, we use a high signal-to-noise version of cameleon, a fluorescent calcium-binding protein, to quantify the activity of the AFD thermosensory neuron of individual worms freely navigating spatial thermal gradients. We find that AFD activity is directly coupled to the worm's exploratory movements in spatial thermal gradients. We show that the worm is able, in principle, to evaluate and guide its own thermotactic behaviors with respect to ambient spatial thermal gradients by monitoring the activity of this single thermosensory neuron.
Figures
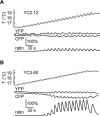
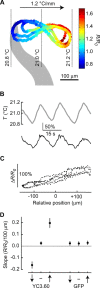
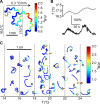
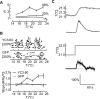
References
-
- Biron D, Shibuya M, Gabel C, Wasserman SM, Clark DA, Brown A, Sengupta P, Samuel AD. A diacylglycerol kinase modulates long-term thermotactic behavioral plasticity in C. elegans. Nat Neurosci. 2006;9:1499–1505. - PubMed
-
- Clark DA, Gabel CV, Lee TM, Samuel AD. Short-term adaptation and temporal processing in the cryophilic response of Caenorhabditis elegans. J Neurophysiol. 2007;97:1903–1910. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
