Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction
- PMID: 17553983
- PMCID: PMC6672152
- DOI: 10.1523/JNEUROSCI.5595-06.2007
Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction
Abstract
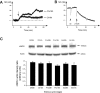
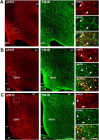
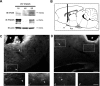
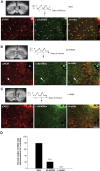
Considerable research has been devoted to the understanding of how nitric oxide (NO) influences brain function. Few studies, however, have addressed how its production is physiologically regulated. Here, we report that protein-protein interactions between neuronal NO synthase (nNOS) and glutamate NMDA receptors via the scaffolding protein postsynaptic density-95 (PSD-95) in the hypothalamic preoptic region of adult female rats is sensitive to cyclic estrogen fluctuation. Coimmunoprecipitation experiments were used to assess the physical association between nNOS and NMDA receptor NR2B subunit in the preoptic region of the hypothalamus. We found that nNOS strongly interacts with NR2B at the onset of the preovulatory surge at proestrus (when estrogen levels are highest) compared with basal-stage diestrous rats. Consistently, estrogen treatment of gonadectomized female rats also increases nNOS/NR2B complex formation. Moreover, endogenous fluctuations in estrogen levels during the estrous cycle coincide with changes in the physical association of nNOS to PSD-95 and the magnitude of NO release in the preoptic region. Finally, temporary and local in vivo suppression of PSD-95 synthesis by using antisense oligodeoxynucleotides leads to inhibition of nNOS activity in the preoptic region and disrupted estrous cyclicity, a process requiring coordinated activation of neurons containing gonadotropin-releasing hormone (the neuropeptide controlling reproductive function). In conclusion, our findings identify a novel steroid-mediated molecular mechanism that enables the adult mammalian brain to control NO release under physiological conditions.
Figures


Similar articles
-
Phosphorylation of N-methyl-D-aspartic acid receptor-associated neuronal nitric oxide synthase depends on estrogens and modulates hypothalamic nitric oxide production during the ovarian cycle.Endocrinology. 2010 Jun;151(6):2723-35. doi: 10.1210/en.2010-0007. Epub 2010 Apr 6. Endocrinology. 2010. PMID: 20371700 Free PMC article.
-
Estradiol induces physical association of neuronal nitric oxide synthase with NMDA receptor and promotes nitric oxide formation via estrogen receptor activation in primary neuronal cultures.J Neurochem. 2009 Apr;109(1):214-24. doi: 10.1111/j.1471-4159.2009.05949.x. Epub 2009 Feb 2. J Neurochem. 2009. PMID: 19187438 Free PMC article.
-
Enhancement of nitric oxide production by association of nitric oxide synthase with N-methyl-D-aspartate receptors via postsynaptic density 95 in genetically engineered Chinese hamster ovary cells: real-time fluorescence imaging using nitric oxide sensitive dye.J Neurochem. 2006 Mar;96(6):1531-9. doi: 10.1111/j.1471-4159.2006.03656.x. Epub 2006 Feb 8. J Neurochem. 2006. PMID: 16464237
-
Nitric oxide as key mediator of neuron-to-neuron and endothelia-to-glia communication involved in the neuroendocrine control of reproduction.Neuroendocrinology. 2011;93(2):74-89. doi: 10.1159/000324147. Epub 2011 Feb 16. Neuroendocrinology. 2011. PMID: 21335953 Review.
-
The PSD-95/nNOS complex: new drugs for depression?Pharmacol Ther. 2012 Feb;133(2):218-29. doi: 10.1016/j.pharmthera.2011.11.005. Epub 2011 Nov 23. Pharmacol Ther. 2012. PMID: 22133842 Review.
Cited by
-
Phenotyping of nNOS neurons in the postnatal and adult female mouse hypothalamus.J Comp Neurol. 2017 Oct 15;525(15):3177-3189. doi: 10.1002/cne.24257. Epub 2017 Jun 19. J Comp Neurol. 2017. PMID: 28577305 Free PMC article.
-
Glutamate neurotransmission from leptin receptor cells is required for typical puberty and reproductive function in female mice.Elife. 2024 Jul 15;13:RP93204. doi: 10.7554/eLife.93204. Elife. 2024. PMID: 39007235 Free PMC article.
-
NOS1 mutations cause hypogonadotropic hypogonadism with sensory and cognitive deficits that can be reversed in infantile mice.Sci Transl Med. 2022 Oct 5;14(665):eabh2369. doi: 10.1126/scitranslmed.abh2369. Epub 2022 Oct 5. Sci Transl Med. 2022. PMID: 36197968 Free PMC article.
-
The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges.Endocr Rev. 2010 Aug;31(4):544-77. doi: 10.1210/er.2009-0023. Epub 2010 Mar 17. Endocr Rev. 2010. PMID: 20237240 Free PMC article. Review.
-
Kisspeptin signaling is required for peripheral but not central stimulation of gonadotropin-releasing hormone neurons by NMDA.J Neurosci. 2010 Jun 23;30(25):8581-90. doi: 10.1523/JNEUROSCI.5486-09.2010. J Neurosci. 2010. PMID: 20573904 Free PMC article.
References
-
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. - PubMed
-
- Beauvillain JC, Tramu G. Immunocytochemical demonstration of LH-RH, somatostatin, and ACTH-like peptide in osmium-postfixed, resin-embedded median eminence. J Histochem Cytochem. 1980;28:1014–1017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
