Nuclear factor I coordinates multiple phases of cerebellar granule cell development via regulation of cell adhesion molecules
- PMID: 17553984
- PMCID: PMC6672151
- DOI: 10.1523/JNEUROSCI.0180-07.2007
Nuclear factor I coordinates multiple phases of cerebellar granule cell development via regulation of cell adhesion molecules
Abstract
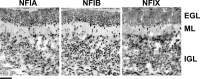
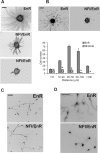
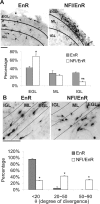
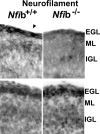
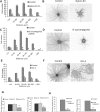
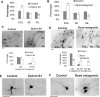
A central question is how various stages of neuronal development are integrated as a differentiation program. Here we show that the nuclear factor I (NFI) family of transcriptional regulators is expressed and functions throughout the postmitotic development of cerebellar granule neurons (CGNs). Expression of an NFI dominant repressor in CGN cultures blocked axon outgrowth and dendrite formation and decreased CGN migration. Inhibition of NFI transactivation also disrupted extension and fasciculation of parallel fibers as well as CGN migration to the internal granule cell layer in cerebellar slices. In postnatal day 17 Nfia-deficient mice, parallel fibers were greatly diminished and disoriented, CGN dendrite formation was dramatically impaired, and migration from the external germinal layer (EGL) was retarded. Axonal marker expression also was disrupted within the EGL of embryonic day 18 Nfib-null mice. NFI regulation of axon extension was observed under conditions of homotypic cell contact, implicating cell surface proteins as downstream mediators of its actions in CGNs. Consistent with this, the cell adhesion molecules ephrin B1 and N-cadherin were identified as NFI gene targets in CGNs using inhibitor and Nfi mutant analysis as well as chromatin immunoprecipitation. Functional inhibition of ephrin B1 or N-cadherin interfered with CGN axon extension and guidance, migration, and dendritogenesis in cell culture as well as in situ. These studies define NFI as a key regulator of postmitotic CGN development, in particular of axon formation, dendritogenesis, and migratory behavior. Furthermore, they reveal how a single transcription factor family can control and integrate multiple aspects of neuronal differentiation through the regulation of cell adhesion molecules.
Figures


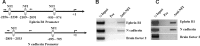
Similar articles
-
Targets of the nuclear factor I regulon involved in early and late development of postmitotic cerebellar granule neurons.J Neurosci Res. 2010 Feb 1;88(2):258-65. doi: 10.1002/jnr.22199. J Neurosci Res. 2010. PMID: 19658195
-
Temporal control of a dendritogenesis-linked gene via REST-dependent regulation of nuclear factor I occupancy.Mol Biol Cell. 2011 Mar 15;22(6):868-79. doi: 10.1091/mbc.E10-10-0817. Epub 2011 Jan 26. Mol Biol Cell. 2011. PMID: 21270437 Free PMC article.
-
Temporal regulation of nuclear factor one occupancy by calcineurin/NFAT governs a voltage-sensitive developmental switch in late maturing neurons.J Neurosci. 2013 Feb 13;33(7):2860-72. doi: 10.1523/JNEUROSCI.3533-12.2013. J Neurosci. 2013. PMID: 23407945 Free PMC article.
-
Differential roles of multiple adhesion molecules in cell migration: granule cell migration in cerebellum.Experientia. 1990 Sep 15;46(9):892-9. doi: 10.1007/BF01939381. Experientia. 1990. PMID: 2209798 Review.
-
Emx and Nfi genes regulate cortical development and axon guidance in the telencephalon.Novartis Found Symp. 2007;288:230-242; discussion 242-5, 276-81. Novartis Found Symp. 2007. PMID: 18494262 Review.
Cited by
-
Nuclear factor one transcription factors in CNS development.Mol Neurobiol. 2009 Feb;39(1):10-23. doi: 10.1007/s12035-008-8048-6. Epub 2008 Dec 5. Mol Neurobiol. 2009. PMID: 19058033
-
Multiple non-cell-autonomous defects underlie neocortical callosal dysgenesis in Nfib-deficient mice.Neural Dev. 2009 Dec 4;4:43. doi: 10.1186/1749-8104-4-43. Neural Dev. 2009. PMID: 19961580 Free PMC article.
-
Consensus Paper: Cerebellar Development.Cerebellum. 2016 Dec;15(6):789-828. doi: 10.1007/s12311-015-0724-2. Cerebellum. 2016. PMID: 26439486 Free PMC article. Review.
-
Alterations in gene expression in the spinal cord of mice lacking Nfix.BMC Res Notes. 2020 Sep 16;13(1):437. doi: 10.1186/s13104-020-05278-w. BMC Res Notes. 2020. PMID: 32938475 Free PMC article.
-
Combined allelic dosage of Nfia and Nfib regulates cortical development.Brain Neurosci Adv. 2017 Nov 22;1:2398212817739433. doi: 10.1177/2398212817739433. eCollection 2017 Jan-Dec. Brain Neurosci Adv. 2017. PMID: 32166136 Free PMC article.
References
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. 3. Maturation of the components of the granular layer. J Comp Neurol. 1972;145:465–513. - PubMed
-
- Barami K, Kirschenbaum B, Lemmon V, Goldman SA. N-cadherin and Ng-CAM/8D9 are involved serially in the migration of newly generated neurons into the adult songbird brain. Neuron. 1994;13:567–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
