UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans
- PMID: 17553987
- PMCID: PMC6672138
- DOI: 10.1523/JNEUROSCI.1466-07.2007
UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans
Abstract
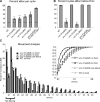
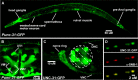
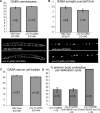
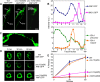
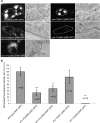
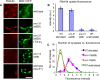
Previous studies indicated that CAPS (calcium-dependent activator protein for secretion) functions as an essential component for the Ca2+-dependent exocytosis of dense-core vesicles in neuroendocrine cells. However, recent mouse knock-out studies suggested an alternative role in the vesicular uptake or storage of catecholamines. To genetically assess the functional role of CAPS, we characterized the sole Caenorhabditis elegans CAPS ortholog UNC-31 (uncoordinated family member) and determined its role in dense-core vesicle-mediated peptide secretion and in synaptic vesicle recycling. Novel assays for dense-core vesicle exocytosis were developed by expressing a prepro-atrial natriuretic factor-green fluorescent protein fusion protein in C. elegans. unc-31 mutants exhibited reduced peptide release in vivo and lacked evoked peptide release in cultured neurons. In contrast, cultured neurons from unc-31 mutants exhibited normal stimulated synaptic vesicle recycling measured by FM4-64 [N-(3-triethylammoniumpropyl)-4-(6-(4-diethylamino)phenyl)hexatrienyl)pyridinium dibromide] dye uptake. Conversely, UNC-13, which exhibits sequence homology to CAPS/UNC-31, was found to be essential for synaptic vesicle but not dense-core vesicle exocytosis. These findings indicate that CAPS/UNC-31 function is not restricted to catecholaminergic vesicles but is generally required for and specific to dense-core vesicle exocytosis. Our results suggest that CAPS/UNC-31 and UNC-13 serve parallel and dedicated roles in dense-core vesicle and synaptic vesicle exocytosis, respectively, in the C. elegans nervous system.
Figures

Similar articles
-
UNC-31/CAPS docks and primes dense core vesicles in C. elegans neurons.Biochem Biophys Res Commun. 2010 Jul 2;397(3):526-31. doi: 10.1016/j.bbrc.2010.05.148. Epub 2010 May 31. Biochem Biophys Res Commun. 2010. PMID: 20515653
-
CAPS and syntaxin dock dense core vesicles to the plasma membrane in neurons.J Cell Biol. 2008 Feb 11;180(3):483-91. doi: 10.1083/jcb.200708018. Epub 2008 Feb 4. J Cell Biol. 2008. PMID: 18250196 Free PMC article.
-
The exocytosis regulator complexin controls spontaneous synaptic vesicle release in a CAPS-dependent manner at C. elegans excitatory synapses.PLoS Biol. 2025 Feb 6;23(2):e3003023. doi: 10.1371/journal.pbio.3003023. eCollection 2025 Feb. PLoS Biol. 2025. PMID: 39913617 Free PMC article.
-
The synaptic vesicle cycle: exocytosis and endocytosis in Drosophila and C. elegans.Curr Opin Neurobiol. 2002 Oct;12(5):499-507. doi: 10.1016/s0959-4388(02)00360-4. Curr Opin Neurobiol. 2002. PMID: 12367628 Review.
-
The Ca(2+)-dependent activator protein for secretion CAPS: do I dock or do I prime?Mol Neurobiol. 2009 Feb;39(1):62-72. doi: 10.1007/s12035-009-8052-5. Epub 2009 Jan 23. Mol Neurobiol. 2009. PMID: 19160073 Review.
Cited by
-
The Vesicle Priming Factor CAPS Functions as a Homodimer via C2 Domain Interactions to Promote Regulated Vesicle Exocytosis.J Biol Chem. 2016 Sep 30;291(40):21257-21270. doi: 10.1074/jbc.M116.728097. Epub 2016 Aug 15. J Biol Chem. 2016. PMID: 27528604 Free PMC article.
-
Behavioral States.Genetics. 2020 Oct;216(2):315-332. doi: 10.1534/genetics.120.303539. Genetics. 2020. PMID: 33023930 Free PMC article. Review.
-
The head mesodermal cell couples FMRFamide neuropeptide signaling with rhythmic muscle contraction in C. elegans.Nat Commun. 2023 Jul 14;14(1):4218. doi: 10.1038/s41467-023-39955-8. Nat Commun. 2023. PMID: 37452027 Free PMC article.
-
Ca2+/CaM binding to CaMKI promotes IMA-3 importin binding and nuclear translocation in sensory neurons to control behavioral adaptation.Elife. 2021 Nov 12;10:e71443. doi: 10.7554/eLife.71443. Elife. 2021. PMID: 34766550 Free PMC article.
-
Loss of the transcriptional repressor PAG-3/Gfi-1 results in enhanced neurosecretion that is dependent on the dense-core vesicle membrane protein IDA-1/IA-2.PLoS Genet. 2009 Apr;5(4):e1000447. doi: 10.1371/journal.pgen.1000447. Epub 2009 Apr 3. PLoS Genet. 2009. PMID: 19343207 Free PMC article.
References
-
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. - PubMed
-
- Ann K, Kowalchyk JA, Loyet KM, Martin TF. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J Biol Chem. 1997;272:19637–19640. - PubMed
-
- Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila UNC-13 is essential for synaptic transmission. Nat Neurosci. 1999;2:965–971. - PubMed
-
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13–1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
