A crucial role for p57(Kip2) in the intracellular timer that controls oligodendrocyte differentiation
- PMID: 17553990
- PMCID: PMC6672145
- DOI: 10.1523/JNEUROSCI.0628-07.2007
A crucial role for p57(Kip2) in the intracellular timer that controls oligodendrocyte differentiation
Abstract
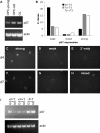
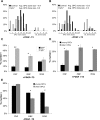
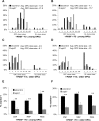
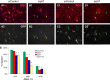
The intracellular molecular mechanism that controls the timing of oligodendrocyte differentiation remains unknown. Temple and Raff (1986) previously showed that an oligodendrocyte precursor cell (OPC) can divide a maximum of approximately eight times before its daughter cells simultaneously cease proliferating and differentiate into oligodendrocytes. They postulated that over time the level of an intracellular molecule might synchronously change in each daughter cell, ultimately reaching a level that prohibited additional proliferation. Here, we report the discovery of such a molecule, the cyclin-dependent kinase inhibitor p57(Kip2) (Cdkn1c). We show in vitro that all daughters of a clone of OPCs express similar levels of p57(Kip2), that p57(Kip2) levels increase over time in proliferating OPCs, and that p57(Kip2) levels regulate how many times an OPC can divide before differentiating. These findings reveal a novel part of the mechanism by which OPCs measure time and are likely to extend to similar timers in many other precursor cell types.
Figures


References
-
- Baas D, Bourbeau D, Sarlieve LL, Ittel ME, Dussault JH, Puymirat J. Oligodendrocyte maturation and progenitor cell proliferation are independently regulated by thyroid hormone. Glia. 1997;19:324–332. - PubMed
-
- Barres BA, Raff MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–942. - PubMed
-
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. - PubMed
-
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
