Role of receptors in Bacillus thuringiensis crystal toxin activity
- PMID: 17554045
- PMCID: PMC1899880
- DOI: 10.1128/MMBR.00034-06
Role of receptors in Bacillus thuringiensis crystal toxin activity
Abstract
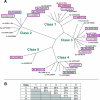
Bacillus thuringiensis produces crystalline protein inclusions with insecticidal or nematocidal properties. These crystal (Cry) proteins determine a particular strain's toxicity profile. Transgenic crops expressing one or more recombinant Cry toxins have become agriculturally important. Individual Cry toxins are usually toxic to only a few species within an order, and receptors on midgut epithelial cells have been shown to be critical determinants of Cry specificity. The best characterized of these receptors have been identified for lepidopterans, and two major receptor classes have emerged: the aminopeptidase N (APN) receptors and the cadherin-like receptors. Currently, 38 different APNs have been reported for 12 different lepidopterans. Each APN belongs to one of five groups that have unique structural features and Cry-binding properties. While 17 different APNs have been reported to bind to Cry toxins, only 2 have been shown to mediate toxin susceptibly in vivo. In contrast, several cadherin-like proteins bind to Cry toxins and confer toxin susceptibility in vitro, and disruption of the cadherin gene has been associated with toxin resistance. Nonetheless, only a small subset of the lepidopteran-specific Cry toxins has been shown to interact with cadherin-like proteins. This review analyzes the interactions between Cry toxins and their receptors, focusing on the identification and validation of receptors, the molecular basis for receptor recognition, the role of the receptor in resistant insects, and proposed models to explain the sequence of events at the cell surface by which receptor binding leads to cell death.
Figures
Similar articles
-
Role of receptor interaction in the mode of action of insecticidal Cry and Cyt toxins produced by Bacillus thuringiensis.Peptides. 2007 Jan;28(1):169-73. doi: 10.1016/j.peptides.2006.06.013. Epub 2006 Dec 4. Peptides. 2007. PMID: 17145116 Review.
-
Cell lines as models for the study of Cry toxins from Bacillus thuringiensis.Insect Biochem Mol Biol. 2018 Feb;93:66-78. doi: 10.1016/j.ibmb.2017.12.008. Epub 2017 Dec 19. Insect Biochem Mol Biol. 2018. PMID: 29269111 Review.
-
A novel aminopeptidase in the fat body of the moth Achaea janata as a receptor for Bacillus thuringiensis Cry toxins and its comparison with midgut aminopeptidase.Biochem J. 2007 Jul 15;405(2):287-97. doi: 10.1042/BJ20070054. Biochem J. 2007. PMID: 17402938 Free PMC article.
-
Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control.Toxicon. 2007 Mar 15;49(4):423-35. doi: 10.1016/j.toxicon.2006.11.022. Epub 2006 Nov 30. Toxicon. 2007. PMID: 17198720 Free PMC article. Review.
-
A high-throughput, in-vitro assay for Bacillus thuringiensis insecticidal proteins.J Biotechnol. 2016 Jan 10;217:72-81. doi: 10.1016/j.jbiotec.2015.10.021. Epub 2015 Oct 30. J Biotechnol. 2016. PMID: 26524384
Cited by
-
A Spodoptera exigua cadherin serves as a putative receptor for Bacillus thuringiensis Cry1Ca toxin and shows differential enhancement of Cry1Ca and Cry1Ac toxicity.Appl Environ Microbiol. 2013 Sep;79(18):5576-83. doi: 10.1128/AEM.01519-13. Epub 2013 Jul 8. Appl Environ Microbiol. 2013. PMID: 23835184 Free PMC article.
-
Resistance to Bacillus thuringiensis Toxin Cry2Ab in Trichoplusia ni Is Conferred by a Novel Genetic Mechanism.Appl Environ Microbiol. 2015 Aug;81(15):5184-95. doi: 10.1128/AEM.00593-15. Epub 2015 May 29. Appl Environ Microbiol. 2015. PMID: 26025894 Free PMC article.
-
Improvement of crystal solubility and increasing toxicity against Caenorhabditis elegans by asparagine substitution in block 3 of Bacillus thuringiensis crystal protein Cry5Ba.Appl Environ Microbiol. 2012 Oct;78(20):7197-204. doi: 10.1128/AEM.01048-12. Epub 2012 Aug 3. Appl Environ Microbiol. 2012. PMID: 22865071 Free PMC article.
-
Cry4Aa and Cry4Ba Mosquito-Active Toxins Utilize Different Domains in Binding to a Particular Culex ALP Isoform: A Functional Toxin Receptor Implicating Differential Actions on Target Larvae.Toxins (Basel). 2022 Sep 21;14(10):652. doi: 10.3390/toxins14100652. Toxins (Basel). 2022. PMID: 36287921 Free PMC article.
-
Differential role of Manduca sexta aminopeptidase-N and alkaline phosphatase in the mode of action of Cry1Aa, Cry1Ab, and Cry1Ac toxins from Bacillus thuringiensis.Appl Environ Microbiol. 2013 Aug;79(15):4543-50. doi: 10.1128/AEM.01062-13. Epub 2013 May 17. Appl Environ Microbiol. 2013. PMID: 23686267 Free PMC article.
References
-
- Aimanova, K. G., M. Zhuang, and S. S. Gill. 2006. Expression of Cry1Ac cadherin receptors in insect midgut and cell lines. J. Invertebr. Pathol. 92:178-187. - PubMed
-
- Angst, B. D., C. Marcozzi, and A. I. Magee. 2001. The cadherin superfamily: diversity in form and function. J. Cell Sci. 114:629-641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

