Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans
- PMID: 17554048
- PMCID: PMC1899878
- DOI: 10.1128/MMBR.00009-06
Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans
Abstract
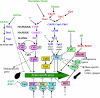
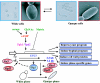
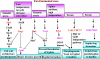
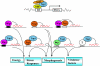
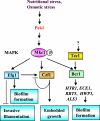
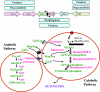
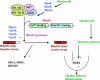
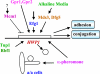
Candida albicans is an opportunistic fungal pathogen that is found in the normal gastrointestinal flora of most healthy humans. However, under certain environmental conditions, it can become a life-threatening pathogen. The shift from commensal organism to pathogen is often correlated with the capacity to undergo morphogenesis. Indeed, under certain conditions, including growth at ambient temperature, the presence of serum or N-acetylglucosamine, neutral pH, and nutrient starvation, C. albicans can undergo reversible transitions from the yeast form to the mycelial form. This morphological plasticity reflects the interplay of various signal transduction pathways, either stimulating or repressing hyphal formation. In this review, we provide an overview of the different sensing and signaling pathways involved in the morphogenesis and pathogenesis of C. albicans. Where appropriate, we compare the analogous pathways/genes in Saccharomyces cerevisiae in an attempt to highlight the evolution of the different components of the two organisms. The downstream components of these pathways, some of which may be interesting antifungal targets, are also discussed.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

