Tomographic digital subtraction angiography for lung perfusion estimation in rodents
- PMID: 17555236
- PMCID: PMC2682230
- DOI: 10.1118/1.2717384
Tomographic digital subtraction angiography for lung perfusion estimation in rodents
Abstract
In vivo measurements of perfusion present a challenge to existing small animal imaging techniques such as magnetic resonance microscopy, micro computed tomography, micro positron emission tomography, and microSPECT, due to combined requirements for high spatial and temporal resolution. We demonstrate the use of tomographic digital subtraction angiography (TDSA) for estimation of perfusion in small animals. TDSA augments conventional digital subtraction angiography (DSA) by providing three-dimensional spatial information using tomosynthesis algorithms. TDSA is based on the novel paradigm that the same time density curves can be reproduced in a number of consecutive injections of microL volumes of contrast at a series of different angles of rotation. The capabilities of TDSA are established in studies on lung perfusion in rats. Using an imaging system developed in-house, we acquired data for four-dimensional (4D) imaging with temporal resolution of 140 ms, in-plane spatial resolution of 100 microm, and slice thickness on the order of millimeters. Based on a structured experimental approach, we optimized TDSA imaging providing a good trade-off between slice thickness, the number of injections, contrast to noise, and immunity to artifacts. Both DSA and TDSA images were used to create parametric maps of perfusion. TDSA imaging has potential application in a number of areas where functional perfusion measurements in 4D can provide valuable insight into animal models of disease and response to therapeutics.
Figures

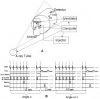



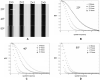


References
-
- Thomas DL. Arterial spin labeling in small animals: Methods and applications to experimental cerebral ischemia. J Magn Reson Imaging. 2005;22:741–744. - PubMed
-
- Benjaminsen IC, Graff BA, Brurberg KG, Rofstad EK. Assessment of tumor blood perfusion by high-resolution dynamic contrast-enhanced mri: A preclinical study of human melanoma xenografts. Magn Reson Med. 2004;52:269–276. - PubMed
-
- Yee SH, Jerabek PA, Fox PT. Non-invasive quantification of cerebral blood flow for rats by micropet imaging of 15o labelled water: The application of a cardiac time-activity curve for the tracer arterial input function. Nucl Med Commun. 2005;26:903–911. - PubMed
-
- Constantinesco A, Choquet P, Monassier L, Israel-Jost V, Mertz L. Assessment of left ventricular perfusion, volumes, and motion in mice using pinhole gated spect. J Nucl Med. 2005;46:1005–1011. - PubMed
-
- Du LY, Lee T-Y, Holdsworth DW. Image quality assessment of a pre-clinical flat-panel volumetric micro-ct scanner. Proc. SPIE Int. Soc. Opt. Eng. 2006:614216.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

