Mutant alpha-galactosidase A enzymes identified in Fabry disease patients with residual enzyme activity: biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin
- PMID: 17555407
- PMCID: PMC1948963
- DOI: 10.1042/BJ20070479
Mutant alpha-galactosidase A enzymes identified in Fabry disease patients with residual enzyme activity: biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin
Abstract
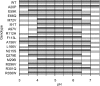
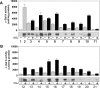
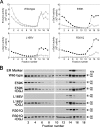
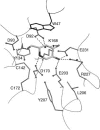
Fabry disease is a lysosomal storage disorder caused by the deficiency of alpha-Gal A (alpha-galactosidase A) activity. In order to understand the molecular mechanism underlying alpha-Gal A deficiency in Fabry disease patients with residual enzyme activity, enzymes with different missense mutations were purified from transfected COS-7 cells and the biochemical properties were characterized. The mutant enzymes detected in variant patients (A20P, E66Q, M72V, I91T, R112H, F113L, N215S, Q279E, M296I, M296V and R301Q), and those found mostly in mild classic patients (A97V, A156V, L166V and R356W) appeared to have normal K(m) and V(max) values. The degradation of all mutants (except E59K) was partially inhibited by treatment with kifunensine, a selective inhibitor of ER (endoplasmic reticulum) alpha-mannosidase I. Metabolic labelling and subcellular fractionation studies in COS-7 cells expressing the L166V and R301Q alpha-Gal A mutants indicated that the mutant protein was retained in the ER and degraded without processing. Addition of DGJ (1-deoxygalactonojirimycin) to the culture medium of COS-7 cells transfected with a large set of missense mutant alpha-Gal A cDNAs effectively increased both enzyme activity and protein yield. DGJ was capable of normalizing intracellular processing of mutant alpha-Gal A found in both classic (L166V) and variant (R301Q) Fabry disease patients. In addition, the residual enzyme activity in fibroblasts or lymphoblasts from both classic and variant hemizygous Fabry disease patients carrying a variety of missense mutations could be substantially increased by cultivation of the cells with DGJ. These results indicate that a large proportion of mutant enzymes in patients with residual enzyme activity are kinetically active. Excessive degradation in the ER could be responsible for the deficiency of enzyme activity in vivo, and the DGJ approach may be broadly applicable to Fabry disease patients with missense mutations.
Figures


References
-
- Brady O. R., Gal A. E., Bradley R. M., Martensson E., Warshaw A. L., Laster L. Enzymatic defect in Fabry's disease: ceramidetrihexosidase deficiency. N. Engl. J. Med. 1967;276:1163–1167. - PubMed
-
- Desnick R. J., Ioannou Y. A., Eng C. M. The Metabolic and Molecular Bases of Inherited Disease. In: Scriver C. R., Beaudet A. L., Sly W. S., Valle D., editors. New York: McGraw-Hill; 2001. pp. 3733–3774.
-
- Shah J. S., Elliott P. M. Fabry disease and the heart: an overview of the natural history and the effect of enzyme replacement therapy. Acta Paediatr. Suppl. 2005;94:11–14. discussion 9–10. - PubMed
-
- Branton M., Schiffmann R., Kopp J. B. Natural history and treatment of renal involvement in Fabry disease. J. Am. Soc. Nephrol. 2002;13(Suppl. 2):S139–S143. - PubMed
-
- Bishop D. F., Grabowski G. A., Desnick R. J. Fabry disease: an asymptomatic hemizygote with significant residual α-galactosidase A activity. Am. J. Hum. Genet. 1981;33:71A.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

