Pharmacokinetics, safety, and efficacy of mycophenolate mofetil in combination with sirolimus or ciclosporin in renal transplant patients
- PMID: 17555465
- PMCID: PMC2198786
- DOI: 10.1111/j.1365-2125.2007.02934.x
Pharmacokinetics, safety, and efficacy of mycophenolate mofetil in combination with sirolimus or ciclosporin in renal transplant patients
Abstract
Aims: To compare the pharmacokinetics of mycophenolic acid (MPA) and its metabolite (MPAG) when mycophenolate mofetil (MMF) is administered in combination with sirolimus or ciclosporin (CsA) in renal allograft recipients. Safety and efficacy (biopsy-proven acute rejection (BPAR)) were also assessed.
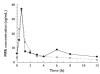
Methods: Patients (n = 45) were randomized 2 : 1 to receive treatment with sirolimus (n = 30; dosed to maintain trough concentrations of 10-25 ng ml(-1) until week 8, and then 8-15 ng ml(-1) thereafter) or CsA (n = 15; administered as per centre practice) both in combination with daclizumab, oral MMF and corticosteroids. Pharmacokinetic assessments were performed at day 7, week 4, and months 3 and 6 post-transplant. The primary endpoint was the AUC(0,12 h) for MPA and MPAG. The pharmacokinetics of sirolimus were also assessed.
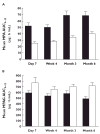
Results: MPA exposure was 39-50% lower (month 6 mean AUC(0,12 h) (95%CI): 40.4 (33.8, 47.0) vs. 68.5 (54.9, 82.0) microg ml(-1) h) and MPAG exposure was 25-52% higher (722 (607, 838) vs. 485 (402, 569) microg ml(-1) h at month 6) in the presence of CsA compared with sirolimus across visits. BPAR was 40.0% with sirolimus and 13.3% with CsA. The incidence of hypertension, tremors and hirsutism was higher with CsA than with sirolimus, while the incidence of diarrhoea, hyperlipidaemia and impaired wound closure was higher with sirolimus. No deaths, malignancies or graft losses were reported.
Conclusions: Co-administration of sirolimus with MMF led to greater MPA exposure, but lower MPAG exposure, than co-administration with CsA. As rejection rates were higher in the absence of CsA, further study of calcineurin inhibitor-free regimens is required before general recommendations can be made.
Figures
References
-
- Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–83. - PubMed
-
- Kaufman DB, Shapiro R, Lucey MR, Cherikh WS, Bustami RT, Dyke D. Immunosuppression: practice and trends. Am J Transplant. 2004;4(Suppl 9):38–53. - PubMed
-
- Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–33. - PubMed
-
- de Mattos AM, Olyaei AJ, Bennett WM. Nephrotoxicity of immunosuppressive drugs. long-term consequences and challenges for the future. Am J Kidney Dis. 2000;35:333–46. - PubMed
-
- Kasiske BL, Tortorice KL, Heim-Duthoy KL, Awni WM, Rao KV. The adverse impact of cyclosporine on serum lipids in renal transplant recipients. Am J Kidney Dis. 1991;17:700–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

