Specificity, promiscuity and localization of ARF protein interactions with NCS-1 and phosphatidylinositol-4 kinase-III beta
- PMID: 17555535
- PMCID: PMC2492389
- DOI: 10.1111/j.1600-0854.2007.00594.x
Specificity, promiscuity and localization of ARF protein interactions with NCS-1 and phosphatidylinositol-4 kinase-III beta
Abstract
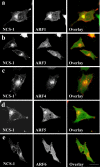
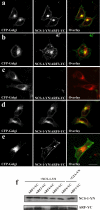
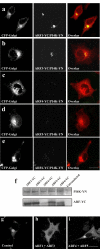
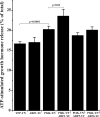
ADP-ribosylation factor (ARF) proteins are involved in multiple intracellular vesicular transport pathways. Most studies have focused on the functions of ARF1 or ARF6 and little is known about the remaining ARF isoforms. Although the mammalian ARF proteins share a high degree of sequence identity, recent evidence has indicated that they may control distinct trafficking steps within cells. A unanswered issue is the degree of specificity of ARF family members for different interacting proteins. To investigate potential functional differences between the human ARF proteins, we have examined the localization of all human ARF isoforms and their interactions with two ARF1 binding proteins, neuronal calcium sensor-1 (NCS-1) and phosphatidylinositol-4 kinase-IIIbeta (PI4Kbeta). Use of a fluorescent protein fragment complementation method showed direct interactions between ARFs 1, 3, 5 and 6 with NCS-1 but at different intracellular locations in live HeLa cells. Photobleaching experiments indicated that complementation did not detect dynamic changes in protein interactions over short-time scales. A more specific interaction between ARFs 1/3 and PI4Kbeta was observed. Consistent with these latter findings ARF1 but not ARF5 or 6 enhanced the stimulatory effect of PI4Kbeta on regulated exocytosis, suggesting a specific role for class-I ARFs in the regulation of PI4Kbeta.
Figures




Similar articles
-
Interaction of neuronal calcium sensor-1 and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol 4-kinase beta and trans-Golgi network-plasma membrane traffic.J Biol Chem. 2005 Feb 18;280(7):6047-54. doi: 10.1074/jbc.M413090200. Epub 2004 Dec 2. J Biol Chem. 2005. PMID: 15576365
-
Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells.J Biol Chem. 2001 Oct 26;276(43):40183-9. doi: 10.1074/jbc.M104048200. Epub 2001 Aug 28. J Biol Chem. 2001. PMID: 11526106
-
Neuronal calcium sensor 1 and phosphatidylinositol 4-OH kinase beta interact in neuronal cells and are translocated to membranes during nucleotide-evoked exocytosis.J Cell Sci. 2002 Oct 15;115(Pt 20):3909-22. doi: 10.1242/jcs.00072. J Cell Sci. 2002. PMID: 12244129
-
Activation of toxin ADP-ribosyltransferases by the family of ADP-ribosylation factors.Adv Exp Med Biol. 1997;419:315-20. doi: 10.1007/978-1-4419-8632-0_41. Adv Exp Med Biol. 1997. PMID: 9193671 Review.
-
Localization and function of Arf family GTPases.Biochem Soc Trans. 2005 Aug;33(Pt 4):639-42. doi: 10.1042/BST0330639. Biochem Soc Trans. 2005. PMID: 16042562 Review.
Cited by
-
Evidence for an interaction between Golli and STIM1 in store-operated calcium entry.Biochem J. 2010 Sep 15;430(3):453-60. doi: 10.1042/BJ20100650. Biochem J. 2010. PMID: 20629634 Free PMC article.
-
Nanomolar affinity of EF-hands in neuronal calcium sensor 1 for bivalent cations Pb2+, Mn2+, and Hg2.Metallomics. 2022 Jul 8;14(7):mfac039. doi: 10.1093/mtomcs/mfac039. Metallomics. 2022. PMID: 35657675 Free PMC article.
-
Defining potential roles of Pb(2+) in neurotoxicity from a calciomics approach.Metallomics. 2016 Jun 1;8(6):563-78. doi: 10.1039/c6mt00038j. Metallomics. 2016. PMID: 27108875 Free PMC article. Review.
-
Neuronal Calcium Sensor-1 Binds the D2 Dopamine Receptor and G-protein-coupled Receptor Kinase 1 (GRK1) Peptides Using Different Modes of Interactions.J Biol Chem. 2015 Jul 24;290(30):18744-56. doi: 10.1074/jbc.M114.627059. Epub 2015 May 15. J Biol Chem. 2015. PMID: 25979333 Free PMC article.
-
Sense and specificity in neuronal calcium signalling.Biochim Biophys Acta. 2015 Sep;1853(9):1921-32. doi: 10.1016/j.bbamcr.2014.10.029. Epub 2014 Nov 4. Biochim Biophys Acta. 2015. PMID: 25447549 Free PMC article. Review.
References
-
- Kahn RA, Volpicelli-Daley L, Bowzard B, Shrivastava-Ranjan P, Li Y, Zhou C, et al. Arf family GTPases: roles in membrane traffic and microtubule dynamics. Biochem Soc Trans. 2005;33:1269–72. - PubMed
-
- Donaldson JG, Honda A. Localization and function of Arf family GTPases. Biochem Soc Trans. 2005;33:639–42. - PubMed
-
- Donaldson JG, Honda A, Weigert R. Multiple activities for Arf1 at the Golgi complex. Biochim. Biophys. Acta. 2005;1744:364–373. - PubMed
-
- Nie Z, Hirsch DS, Randazzo PA. Arf and its many interactors. Curr. Biol. 2003;15:396–404. - PubMed
-
- Godi A, Pertile P, Meyers R, Marra P, DiTullio G, Lurisci C, et al. ARF mediates recruitment of PtdIns-4-OH kinase-B and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

