Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance
- PMID: 17555547
- PMCID: PMC3600610
- DOI: 10.1111/j.1471-4159.2007.04675.x
Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance
Abstract
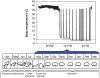
Torpor during hibernation defines the nadir of mammalian metabolism where whole animal rates of metabolism are decreased to as low as 2% of basal metabolic rate. This capacity to decrease profoundly the metabolic demand of organs and tissues has the potential to translate into novel therapies for the treatment of ischemia associated with stroke, cardiac arrest or trauma where delivery of oxygen and nutrients fails to meet demand. If metabolic demand could be arrested in a regulated way, cell and tissue injury could be attenuated. Metabolic suppression achieved during hibernation is regulated, in part, by the central nervous system through indirect and possibly direct means. In this study, we review recent evidence for mechanisms of central nervous system control of torpor in hibernating rodents including evidence of a permissive, hibernation protein complex, a role for A1 adenosine receptors, mu opiate receptors, glutamate and thyrotropin-releasing hormone. Central sites for regulation of torpor include the hippocampus, hypothalamus and nuclei of the autonomic nervous system. In addition, we discuss evidence that hibernation phenotypes can be translated to non-hibernating species by H(2)S and 3-iodothyronamine with the caveat that the hypothermia, bradycardia, and metabolic suppression induced by these compounds may or may not be identical to mechanisms employed in true hibernation.
Figures
Similar articles
-
Metabolic rate depression: the biochemistry of mammalian hibernation.Adv Clin Chem. 2010;52:77-108. Adv Clin Chem. 2010. PMID: 21275340 Review.
-
The Central Control of Energy Expenditure: Exploiting Torpor for Medical Applications.Annu Rev Physiol. 2017 Feb 10;79:167-186. doi: 10.1146/annurev-physiol-022516-034133. Epub 2016 Oct 28. Annu Rev Physiol. 2017. PMID: 27813827 Review.
-
The role of energy availability in Mammalian hibernation: a cost-benefit approach.Physiol Biochem Zool. 2003 Mar-Apr;76(2):165-79. doi: 10.1086/367950. Physiol Biochem Zool. 2003. PMID: 12794670 Review.
-
Central adenosine receptor signaling is necessary for daily torpor in mice.Am J Physiol Regul Integr Comp Physiol. 2012 Sep 1;303(5):R477-84. doi: 10.1152/ajpregu.00081.2012. Epub 2012 Jul 11. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22785425
-
[Pharmacological aspects of mammalian hibernation: central thermoregulation factors in hibernation cycle].Nihon Yakurigaku Zasshi. 2000 Nov;116(5):304-12. doi: 10.1254/fpj.116.304. Nihon Yakurigaku Zasshi. 2000. PMID: 11215381 Review. Japanese.
Cited by
-
Preconditioning the brain: moving on to the next frontier of neurotherapeutics.Stroke. 2012 Jun;43(6):1455-7. doi: 10.1161/STROKEAHA.111.646919. Epub 2012 Mar 29. Stroke. 2012. PMID: 22461331 Free PMC article. No abstract available.
-
Hypoxia, hibernation and Neuroprotection: An Experimental Study in Mice.Aging Dis. 2018 Aug 1;9(4):761-768. doi: 10.14336/AD.2018.0702. eCollection 2018 Aug. Aging Dis. 2018. PMID: 30090664 Free PMC article.
-
Identification of qRT-PCR reference genes for analysis of opioid gene expression in a hibernator.J Comp Physiol B. 2010 Apr;180(4):619-29. doi: 10.1007/s00360-009-0430-9. Epub 2009 Dec 23. J Comp Physiol B. 2010. PMID: 20033416
-
Seasonal oscillation of liver-derived hibernation protein complex in the central nervous system of non-hibernating mammals.J Exp Biol. 2014 Aug 1;217(Pt 15):2667-79. doi: 10.1242/jeb.095976. J Exp Biol. 2014. PMID: 25079892 Free PMC article.
-
Omega 3 fatty acids stimulate thermogenesis during torpor in the Arctic Ground Squirrel.Sci Rep. 2021 Jan 14;11(1):1340. doi: 10.1038/s41598-020-78763-8. Sci Rep. 2021. PMID: 33446684 Free PMC article.
References
-
- Alam N, Szymusiak R, McGinty D. Local preoptic/anterior hypothalamic warming alters spontaneous and evoked neuronal activity in the magno-cellular basal forebrain. Brain Res. 1995;696:221–230. - PubMed
-
- Alanko L, Porkka-Heiskanen T, Soinila S. Localization of equilibrative nucleoside transporters in the rat brain. J. Chem. Neuroanat. 2006;31:162–168. - PubMed
-
- Arrigoni E, Chamberlin NL, Saper CB, McCarley RW. Adenosine inhibits basal forebrain cholinergic and noncholinergic neurons in vitro. Neuroscience. 2006;140:403–413. - PubMed
-
- Barnes BM, Kretzmann M, Zucker I, Licht P. Plasma androgen and gonadotropin levels during hibernation and testicular maturation in golden-mantled ground squirrels. Biol. Reprod. 1988;38:616–622. - PubMed
-
- Barros RC, Branco LG. Role of central adenosine in the respiratory and thermoregulatory responses to hypoxia. Neuroreport. 2000;11:193–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

