Cytokine responses of CD4+ T cells during a Plasmodium chabaudi chabaudi (ER) blood-stage infection in mice initiated by the natural route of infection
- PMID: 17555592
- PMCID: PMC1904224
- DOI: 10.1186/1475-2875-6-77
Cytokine responses of CD4+ T cells during a Plasmodium chabaudi chabaudi (ER) blood-stage infection in mice initiated by the natural route of infection
Abstract
Background: Investigation of host responses to blood stages of Plasmodium spp, and the immunopathology associated with this phase of the life cycle are often performed on mice infected directly with infected red blood cells. Thus, the effects of mosquito bites and the pre-erythrocytic stages of the parasite, which would be present in natural infection, are ignored In this paper, Plasmodium chabaudi chabaudi infections of mice injected directly with infected red blood cells were compared with those of mice infected by the bites of infected mosquitoes, in order to determine whether the courses of primary infection and splenic CD4 T cell responses are similar.
Methods: C57Bl/6 mice were injected with red blood cells infected with P. chabaudi (ER) or infected via the bite of Anopheles stephensi mosquitoes. Parasitaemia were monitored by Giemsa-stained thin blood films. Total spleen cells, CD4+ T cells, and cytokine production (IFN-gamma, IL-2, IL-4, IL-10) were analysed by flow cytometry. In some experiments, mice were subjected to bites of uninfected mosquitoes prior to infectious bites in order to determine whether mosquito bites per se could affect a subsequent P. chabaudi infection.
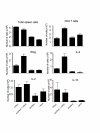
Results: P. chabaudi (ER) infections initiated by mosquito bite were characterized by lower parasitaemia of shorter duration than those observed after direct blood challenge. However, splenomegaly was comparable suggesting that parasitaemia alone does not account for the increase in spleen size. Total numbers of CD4 T cells and those producing IFN-gamma, IL-10 and IL-2 were reduced in comparison to direct blood challenge. By contrast, the reduction in IL-4 producing cells was less marked suggesting that there is a proportionally lower Th1-like response in mice infected via infectious mosquitoes. Strikingly, pre-exposure to bites of uninfected mosquitoes reduced the magnitude and duration of the subsequent mosquito-transmitted infection still further, but enhanced the response of CD4 T cells producing IFN-gamma and IL-4.
Conclusion: The data in this paper suggest that studying early host responses in blood stage malaria infections measured after direct blood challenge of mice may not completely reflect the natural situation, and more detailed investigations of blood-stage immunity after mosquito transmission in experimental models should be considered.
Figures



Similar articles
-
The spleen CD4+ T cell response to blood-stage Plasmodium chabaudi malaria develops in two phases characterized by different properties.PLoS One. 2011;6(7):e22434. doi: 10.1371/journal.pone.0022434. Epub 2011 Jul 21. PLoS One. 2011. PMID: 21814579 Free PMC article.
-
Modulation of experimental blood stage malaria through blockade of the B7/CD28 T-cell costimulatory pathway.Immunology. 1999 Mar;96(3):498-504. doi: 10.1046/j.1365-2567.1999.00718.x. Immunology. 1999. PMID: 10233733 Free PMC article.
-
The influence of gammadelta T cells on the CD4+ T cell and antibody response during a primary Plasmodium chabaudi chabaudi infection in mice.Parasite Immunol. 2002 Mar;24(3):131-40. doi: 10.1046/j.1365-3024.2002.00446.x. Parasite Immunol. 2002. PMID: 11982858
-
The response of CD4+ T cells to Plasmodium chabaudi chabaudi.Immunol Rev. 1989 Dec;112:71-94. doi: 10.1111/j.1600-065x.1989.tb00553.x. Immunol Rev. 1989. PMID: 2575075 Review.
-
Dendritic cells, pro-inflammatory responses, and antigen presentation in a rodent malaria infection.Immunol Rev. 2004 Oct;201:35-47. doi: 10.1111/j.0105-2896.2004.00182.x. Immunol Rev. 2004. PMID: 15361231 Review.
Cited by
-
Changes in cell migration-related molecules expressed by thymic microenvironment during experimental Plasmodium berghei infection: consequences on thymocyte development.Immunology. 2010 Feb;129(2):248-56. doi: 10.1111/j.1365-2567.2009.03177.x. Epub 2009 Sep 9. Immunology. 2010. PMID: 19824923 Free PMC article.
-
An Erythrocyte Membrane-Associated Antigen, PvTRAg-26 of Plasmodium vivax: A Study of Its Antigenicity and Immunogenicity.Front Public Health. 2020 Apr 28;8:148. doi: 10.3389/fpubh.2020.00148. eCollection 2020. Front Public Health. 2020. PMID: 32411650 Free PMC article.
-
The contribution of Plasmodium chabaudi to our understanding of malaria.Trends Parasitol. 2012 Feb;28(2):73-82. doi: 10.1016/j.pt.2011.10.006. Epub 2011 Nov 17. Trends Parasitol. 2012. PMID: 22100995 Free PMC article. Review.
-
Mosquito transmission of the rodent malaria parasite Plasmodium chabaudi.Malar J. 2012 Dec 6;11:407. doi: 10.1186/1475-2875-11-407. Malar J. 2012. PMID: 23217144 Free PMC article.
-
Caspase-1 activation of interleukin-1β (IL-1β) and IL-18 is dispensable for induction of experimental cerebral malaria.Infect Immun. 2011 Sep;79(9):3633-41. doi: 10.1128/IAI.05459-11. Epub 2011 Jun 27. Infect Immun. 2011. PMID: 21708993 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

