Differentiation of xenografted human fetal lung parenchyma
- PMID: 17555893
- PMCID: PMC2753467
- DOI: 10.1016/j.earlhumdev.2007.04.002
Differentiation of xenografted human fetal lung parenchyma
Abstract
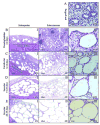
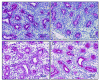
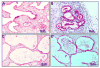
The goal of this study was to characterize xenografted human fetal lung tissue with respect to developmental stage-specific cytodifferentiation. Human fetal lung tissue (pseudoglandular stage) was grafted either beneath the renal capsule or the skin of athymic mice (NCr-nu). Tissues were analyzed from 3 to 42 days post-engraftment for morphological alterations by light and electron microscopy (EM), and for surfactant protein mRNA and protein by reverse transcription-polymerase chain reaction (RT-PCR) and immunocytochemistry (ICC), respectively. The changes observed resemble those seen in human lung development in utero in many respects, including the differentiation of epithelium to the saccular stage. Each stage occurred over approximately one week in the graft in contrast to the eight weeks of normal in utero development. At all time points examined, all four surfactant proteins (SP-A, SP-B, SP-C, and SP-D) were detected in the epithelium by ICC. Lamellar bodies were first identified by EM in 14 day xenografts. By day 21, a significant increase in lamellar body expression was observed. Cellular proliferation, as marked by PCNA ICC and elastic fiber deposition resembled those of canalicular and saccular in utero development. This model in which xenografted lung tissue in different stages of development is available may facilitate the study of human fetal lung development and the impact of various pharmacological agents on this process.
Figures





References
-
- Alcorn JL, Mendelson CR. Trafficking of surfactant protein A in fetal rabbit lung in organ culture. Am J Physiol. 1993;264:L27–35. - PubMed
-
- Ballard PL. Combined hormonal treatment and lung maturation. Semin Perinatol. 1984;8:283–292. - PubMed
-
- Ballard PL, Nogee LM, Beers MF, Ballard RA, Planer BC, Polk L, deMello DE, Moxley MA, Longmore WJ. Partial deficiency of surfactant protein B in an infant with chronic lung disease. Pediatrics. 1995;96:1046–1052. - PubMed
-
- Brown C. Antigen retrieval methods for immunohistochemistry. Toxicol Pathol. 1998;26:830–831. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

