Glucocorticoids and insulin both modulate caloric intake through actions on the brain
- PMID: 17556388
- PMCID: PMC2277039
- DOI: 10.1113/jphysiol.2007.136051
Glucocorticoids and insulin both modulate caloric intake through actions on the brain
Abstract
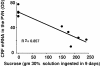
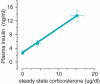
Glucocorticoids act primarily in a feed-forward fashion on brain to activate CNS pathways that implement wanting appropriate to physiological needs. Thus, depending on the available conditions, elevated glucocorticoids may augment the behavioural want to run, fight or feed. Although glucocorticoids stimulate intake of chow, fat and sucrose, insulin appears to sculpt calorie-associated desires toward foods high in fat, acting through hepatic branch afferents of the vagus nerve. Both conditions of reduced food allowance and chronic stress excite glucocorticoid-augmented central neural networks that may lead toward ultimate abdominal obesity.
Figures
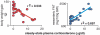

Comment in
-
Obesity and the central nervous system.J Physiol. 2007 Sep 1;583(Pt 2):423. doi: 10.1113/jphysiol.2007.140566. J Physiol. 2007. PMID: 17766646 Free PMC article. No abstract available.
References
-
- Akana SF, Cascio CS, Shinsako J, Dallman MF. Corticosterone: narrow range required for normal body and thymus weight and ACTH. Am J Physiol Regul Integr Comp Physiol. 1985;249:R527–R532. - PubMed
-
- Akana S, Scribner K, Bradbury M, Strack A, Walker C-D, Dallman M. Feedback sensitivity of the rat hypothalamo-pituitary-adrenal axis and its capacity to adjust to exogenous corticosterone. Endocrinology. 1992;131:585–594. - PubMed
-
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–979. - PubMed
-
- Bell ME, Bhargava A, Soriano L, Laugero K, Akana SF, Dallman MF. Sucrose intake and corticosterone interact with cold to modulate behavior, energy balance, autonomic outflow and neuroendocrine responses during chronic stress. J Neuroendocrinol. 2002;14:330–342. - PubMed
-
- Bell ME, Bhatnagar S, Liang J, Soriano L, Nagy TR, Dallman MF. Voluntary sucrose ingestion, like corticosterone replacement, prevents the metabolic deficits of adrenalectomy. J Neuroendocrinol. 2000;12:461–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

