Crucial role of the protein C pathway in governing microvascular inflammation in inflammatory bowel disease
- PMID: 17557119
- PMCID: PMC1884689
- DOI: 10.1172/JCI31027
Crucial role of the protein C pathway in governing microvascular inflammation in inflammatory bowel disease
Abstract
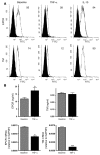
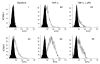
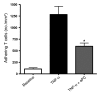
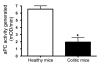
Endothelial protein C receptor (EPCR) and thrombomodulin (TM) are expressed at high levels in the resting microvasculature and convert protein C (PC) into its activated form, which is a potent anticoagulant and antiinflammatory molecule. Here we provide evidence that in Crohn disease (CD) and ulcerative colitis (UC), the 2 major forms of inflammatory bowel disease (IBD), there was loss of expression of endothelial EPCR and TM, which in turns caused impairment of PC activation by the inflamed mucosal microvasculature. In isolated human intestinal endothelial cells, administration of recombinant activated PC had a potent antiinflammatory effect, as demonstrated by downregulated cytokine-dependent cell adhesion molecule expression and chemokine production as well as inhibited leukocyte adhesion. In vivo, administration of activated PC was therapeutically effective in ameliorating experimental colitis as evidenced by reduced weight loss, disease activity index, and histological colitis scores as well as inhibited leukocyte adhesion to the inflamed intestinal vessels. The results suggest that the PC pathway represents a new system crucially involved in governing intestinal homeostasis mediated by the mucosal microvasculature. Restoring the PC pathway may represent a new therapeutic approach to suppress intestinal inflammation in IBD.
Figures






Similar articles
-
The protein C pathway in inflammatory bowel disease: the missing link between inflammation and coagulation.Trends Mol Med. 2008 Jun;14(6):237-44. doi: 10.1016/j.molmed.2008.03.005. Epub 2008 May 3. Trends Mol Med. 2008. PMID: 18457995
-
A novel beta-oxa polyunsaturated fatty acid downregulates the activation of the IkappaB kinase/nuclear factor kappaB pathway, inhibits expression of endothelial cell adhesion molecules, and depresses inflammation.Circ Res. 2006 Jul 7;99(1):34-41. doi: 10.1161/01.RES.0000231292.66084.cd. Epub 2006 Jun 8. Circ Res. 2006. PMID: 16763165
-
VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis.Gastroenterology. 2009 Feb;136(2):585-95.e5. doi: 10.1053/j.gastro.2008.09.064. Epub 2008 Oct 7. Gastroenterology. 2009. PMID: 19013462
-
Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues.Am J Physiol Gastrointest Liver Physiol. 2007 Jul;293(1):G5-G18. doi: 10.1152/ajpgi.00107.2007. Epub 2007 Apr 26. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17463183 Review.
-
Crosstalk between inflammation and thrombosis.Maturitas. 2004 Apr 15;47(4):305-14. doi: 10.1016/j.maturitas.2003.10.015. Maturitas. 2004. PMID: 15063484 Review.
Cited by
-
Hyaluronan Accelerates Intestinal Mucosal Healing through Interaction with TSG-6.Cells. 2019 Sep 12;8(9):1074. doi: 10.3390/cells8091074. Cells. 2019. PMID: 31547322 Free PMC article.
-
Inhibitory effects of rutin on the endothelial protein C receptor shedding in vitro and in vivo.Inflammation. 2014 Oct;37(5):1424-31. doi: 10.1007/s10753-014-9866-5. Inflammation. 2014. PMID: 24622777
-
Endothelium--role in regulation of coagulation and inflammation.Semin Immunopathol. 2012 Jan;34(1):93-106. doi: 10.1007/s00281-011-0285-5. Epub 2011 Aug 4. Semin Immunopathol. 2012. PMID: 21845431 Free PMC article. Review.
-
Sam68 contributes to intestinal inflammation in experimental and human colitis.Cell Mol Life Sci. 2021 Dec;78(23):7635-7648. doi: 10.1007/s00018-021-03976-7. Epub 2021 Oct 24. Cell Mol Life Sci. 2021. PMID: 34693458 Free PMC article.
-
Platelet abnormalities during colonic inflammation.Inflamm Bowel Dis. 2013 May;19(6):1245-53. doi: 10.1097/MIB.0b013e318281f3df. Inflamm Bowel Dis. 2013. PMID: 23518812 Free PMC article.
References
-
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. - PubMed
-
- Podolsky D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002;347:417–429. - PubMed
-
- Hatoum O.A., Binion D.G. The vasculature and inflammatory bowel disease: contribution to pathogenesis and clinical pathology. Inflamm. Bowel Dis. 2005;11:304–313. - PubMed
-
- Fiocchi C. Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am. J. Physiol. 1997;273:G769–G775. - PubMed
-
- Fiocchi, C., Ina, K., Danese, S., Leite, A., and Vogel, J.D. 2003. Alterations of the mucosal immune system in IBD mesenchymal and endothelial cells. In Immune mechanisms in inflammatory bowel disease. Landes Bioscience Publishers. New York, New York, USA. 168–176.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

