The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo
- PMID: 17557122
- PMCID: PMC1884686
- DOI: 10.1172/JCI29877
The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo
Abstract
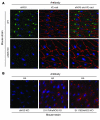
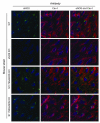
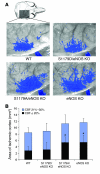
NO plays critical roles in vascular function. We show that modulation of the eNOS serine 1179 (S1179) phosphorylation site affects vascular reactivity and determines stroke size in vivo. Transgenic mice expressing only a phosphomimetic (S1179D) form of eNOS show greater vascular reactivity, develop less severe strokes, and have improved cerebral blood flow in a middle cerebral artery occlusion model than mice expressing an unphosphorylatable (S1179A) form. These results provide a molecular mechanism by which multiple diverse cardiovascular risks, such as diabetes and obesity, may be centrally integrated by eNOS phosphorylation in vivo to influence blood flow and cardiovascular disease. They also demonstrate the in vivo relevance of posttranslational modification of eNOS in vascular function.
Figures



References
-
- Bredt D.S., Snyder S.H. Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem. 1994;63:175–195. - PubMed
-
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993;329:2002–2012. - PubMed
-
- Dudzinski D.M., Igarashi J., Greif D., Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharmacol. Toxicol. 2006;46:235–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

