Pigment spectra and intermolecular interaction potentials in glasses and proteins
- PMID: 17557783
- PMCID: PMC1965442
- DOI: 10.1529/biophysj.107.104273
Pigment spectra and intermolecular interaction potentials in glasses and proteins
Abstract
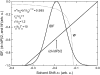
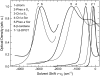
A model is proposed for chromophore optical spectra in solids over a wide range of temperatures and pressures. Inhomogeneous band shapes and their pressure dependence, as well as baric shift coefficients of spectral lines, selected by the frequency, were derived using Lennard-Jones potentials of the ground and excited states. Quadratic electron-phonon coupling constants, describing the thermal shift and broadening of zero-phonon lines, were also calculated. Experimentally, thermal shift and broadening of spectral holes were studied between 5 and 40 K for a synthetic pigment, chlorin, embedded in polymer hosts. The baric effects on holes were determined by applying hydrostatic He gas pressure up to 200 bar, at 6 K. Absorption spectra of pheophytin a, chlorophyll a, and beta-carotene in polymers and plant photosystem II CP47 complex were measured between 5 (or 77) and 300 K, and subject to Voigtian deconvolution. A narrowing of inhomogeneous bandwidth with increasing temperature, predicted on the basis of hole behavior, was observed as the shrinking of Gaussian spectral component. The Lorentzian broadening was ascribed to optical dephasing up to 300 K in transitions with weak to moderate linear electron-phonon coupling strength. The thermal broadening is purely Gaussian in multiphonon transitions (S(2) band of beta-carotene, Soret bands of tetrapyrrolic pigments), and the Lorentz process appears to be suppressed, indicating a lack of exponential dephasing. Density, polarity, polarizability, compressibility, and other local parameters of the pigment binding sites in biologically relevant systems can be deduced from spectroscopic data, provided that sufficient background information is available.
Figures










References
-
- Haugland, R. P. 2002. Handbook of Fluorescent Probes and Research Chemicals, 9th ed. Molecular Probes, Eugene, OR. http://probes.invitrogen.com/handbook.
-
- Fritsch, C., and T. Ruzicka. 2003. Fluorescence Diagnosis and Photodynamic Therapy of Skin Diseases. Springer, New York.
-
- K. Sullivan and S. Kay, editors. 1999. Green Fluorescent Proteins. Academic Press, San Diego.
-
- van Amerongen, H., L. Valkunas, and R. van Grondelle. 2000. Photosynthetic Excitons. World Scientific, Singapore.
-
- Mataga, N., and T. Kubota. 1970. Molecular Interactions and Electronic Spectra. Marcel Dekker, New York.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

