Mutations in the tryptophan operon allow PurF-independent thiamine synthesis by altering flux in vivo
- PMID: 17557816
- PMCID: PMC2223571
- DOI: 10.1128/JB.00582-07
Mutations in the tryptophan operon allow PurF-independent thiamine synthesis by altering flux in vivo
Abstract
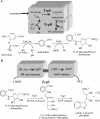
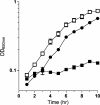
Phosphoribosyl amine (PRA) is an intermediate in purine biosynthesis and also required for thiamine biosynthesis in Salmonella enterica. PRA is normally synthesized by phosphoribosyl pyrophosphate amidotransferase, a high-turnover enzyme of the purine biosynthetic pathway encoded by purF. However, PurF-independent PRA synthesis has been observed in strains having different genetic backgrounds and growing under diverse conditions. Genetic analysis has shown that the anthranilate synthase-phosphoribosyltransferase (AS-PRT) enzyme complex, involved in the synthesis of tryptophan, can play a role in the synthesis of PRA. This work describes the in vitro synthesis of PRA in the presence of the purified components of the AS-PRT complex. Results from in vitro assays and in vivo studies indicate that the cellular accumulation of phosphoribosyl anthranilate can result in nonenzymatic PRA formation sufficient for thiamine synthesis. These studies have uncovered a mechanism used by cells to redistribute metabolites to ensure thiamine synthesis and may define a general paradigm of metabolic robustness.
Figures



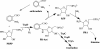


Similar articles
-
Anthranilate phosphoribosyl transferase (TrpD) generates phosphoribosylamine for thiamine synthesis from enamines and phosphoribosyl pyrophosphate.ACS Chem Biol. 2013 Jan 18;8(1):242-8. doi: 10.1021/cb300364k. Epub 2012 Nov 2. ACS Chem Biol. 2013. PMID: 23101964 Free PMC article.
-
PurF-independent phosphoribosyl amine formation in yjgF mutants of Salmonella enterica utilizes the tryptophan biosynthetic enzyme complex anthranilate synthase-phosphoribosyltransferase.J Bacteriol. 2006 Oct;188(19):6786-92. doi: 10.1128/JB.00745-06. J Bacteriol. 2006. PMID: 16980480 Free PMC article.
-
Anthranilate synthase can generate sufficient phosphoribosyl amine for thiamine synthesis in Salmonella enterica.J Bacteriol. 2003 Sep;185(17):5125-32. doi: 10.1128/JB.185.17.5125-5132.2003. J Bacteriol. 2003. PMID: 12923085 Free PMC article.
-
An Unexpected Route to an Essential Cofactor: Escherichia coli Relies on Threonine for Thiamine Biosynthesis.mBio. 2016 Jan 5;7(1):e01840-15. doi: 10.1128/mBio.01840-15. mBio. 2016. PMID: 26733068 Free PMC article.
-
Structure, mechanism and inhibition of anthranilate phosphoribosyltransferase.Philos Trans R Soc Lond B Biol Sci. 2023 Feb 27;378(1871):20220039. doi: 10.1098/rstb.2022.0039. Epub 2023 Jan 11. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 36633281 Free PMC article. Review.
Cited by
-
The three-legged stool of understanding metabolism: integrating metabolomics with biochemical genetics and computational modeling.AIMS Microbiol. 2018 Apr 8;4(2):289-303. doi: 10.3934/microbiol.2018.2.289. eCollection 2018. AIMS Microbiol. 2018. PMID: 31294216 Free PMC article.
-
Perturbations in histidine biosynthesis uncover robustness in the metabolic network of Salmonella enterica.PLoS One. 2012;7(10):e48207. doi: 10.1371/journal.pone.0048207. Epub 2012 Oct 25. PLoS One. 2012. PMID: 23133571 Free PMC article.
-
A study in molecular contingency: glutamine phosphoribosylpyrophosphate amidotransferase is a promiscuous and evolvable phosphoribosylanthranilate isomerase.J Mol Biol. 2008 Mar 21;377(2):323-36. doi: 10.1016/j.jmb.2008.01.043. Epub 2008 Jan 26. J Mol Biol. 2008. PMID: 18272177 Free PMC article.
-
Anthranilate phosphoribosyl transferase (TrpD) generates phosphoribosylamine for thiamine synthesis from enamines and phosphoribosyl pyrophosphate.ACS Chem Biol. 2013 Jan 18;8(1):242-8. doi: 10.1021/cb300364k. Epub 2012 Nov 2. ACS Chem Biol. 2013. PMID: 23101964 Free PMC article.
-
Phosphoribosylpyrophosphate synthetase (PrsA) variants alter cellular pools of ribose 5-phosphate and influence thiamine synthesis in Salmonella enterica.Microbiology (Reading). 2010 Mar;156(Pt 3):950-959. doi: 10.1099/mic.0.033050-0. Epub 2009 Dec 3. Microbiology (Reading). 2010. PMID: 19959576 Free PMC article.
References
-
- Bauerle, R. H., and P. Margolin. 1966. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudo polarity mutations. Cold Spring Harbor Symp. Quant. Biol. 31:203-214. - PubMed
-
- Caligiuri, M. G., and R. Bauerle. 1991. Identification of amino acid residues involved in feedback regulation of the anthranilate synthase complex from Salmonella typhimurium. Evidence for an amino-terminal regulatory site. J. Biol. Chem. 2668328-8335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

