The effect of the palmitoylethanolamide analogue, palmitoylallylamide (L-29) on pain behaviour in rodent models of neuropathy
- PMID: 17558434
- PMCID: PMC2042941
- DOI: 10.1038/sj.bjp.0707326
The effect of the palmitoylethanolamide analogue, palmitoylallylamide (L-29) on pain behaviour in rodent models of neuropathy
Abstract
Background and purpose: Cannabinoids are associated with analgesia in acute and chronic pain states. A spectrum of central cannabinoid (CB(1)) receptor-mediated motor and psychotropic side effects limit their therapeutic potential. Here, we investigate the analgesic effect of the palmitoylethanolamide (PEA) analogue, palmitoylallylamide (L-29), which via inhibition of fatty acid amide hydrolase (FAAH) may potentiate endocannabinoids thereby avoiding psychotropic side effects.
Experimental approach: The in vivo analysis of the effect of L-29 on measures of pain behaviour in three rat models of neuropathic pain.
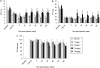
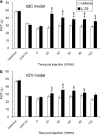
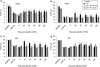
Key results: Systemically administered L-29 (10 mg kg(-1)) reduced hypersensitivity to mechanical and thermal stimuli in the partial sciatic nerve injury (PSNI) model of neuropathic pain; and mechanical hypersensitivity in a model of antiretroviral (ddC)-associated hypersensitivity and a model of varicella zoster virus (VZV)-associated hypersensitivity. The effects of L-29 were comparable to those of gabapentin (50 mg kg(-1)). The CB(1) receptor antagonist SR141716a (1 mg kg(-1)) and the CB(2) receptor antagonist SR144528 (1 mg kg(-1)) reduced the effect of L-29 on hypersensitivity in the PSNI and ddC models, but not in the VZV model. The peroxisome proliferator-activated receptor-alpha antagonist, MK-886 (1 mg kg(-1)), partially attenuated the effect of L-29 on hypersensitivity in the PSNI model. L-29 (10 mg kg(-1)) significantly attenuated thigmotactic behaviour in the open field arena without effect on locomotor activity.
Conclusions and implications: L-29 produces analgesia in a range of neuropathic pain models. This presents L-29 as a novel analgesic compound that may target the endogenous cannabinoid system while avoiding undesirable side effects associated with direct cannabinoid receptor activation.
Figures




Similar articles
-
Attenuation of persistent pain-related behavior by fatty acid amide hydrolase (FAAH) inhibitors in a rat model of HIV sensory neuropathy.Neuropharmacology. 2015 Aug;95:100-9. doi: 10.1016/j.neuropharm.2014.11.024. Epub 2014 Dec 5. Neuropharmacology. 2015. PMID: 25486617 Free PMC article.
-
Inhibitory effect of anandamide on resiniferatoxin-induced sensory neuropeptide release in vivo and neuropathic hyperalgesia in the rat.Life Sci. 2003 Sep 19;73(18):2345-53. doi: 10.1016/s0024-3205(03)00651-9. Life Sci. 2003. PMID: 12941436
-
Non-cannabinoid CB1, non-cannabinoid CB2 antinociceptive effects of several novel compounds in the PPQ stretch test in mice.Eur J Pharmacol. 2006 Sep 28;546(1-3):60-8. doi: 10.1016/j.ejphar.2006.07.024. Epub 2006 Jul 25. Eur J Pharmacol. 2006. PMID: 16919265
-
Palmitoylethanolamide and Its Biobehavioral Correlates in Autism Spectrum Disorder: A Systematic Review of Human and Animal Evidence.Nutrients. 2021 Apr 18;13(4):1346. doi: 10.3390/nu13041346. Nutrients. 2021. PMID: 33919499 Free PMC article.
-
The palmitoylethanolamide and oleamide enigmas : are these two fatty acid amides cannabimimetic?Curr Med Chem. 1999 Aug;6(8):757-73. Curr Med Chem. 1999. PMID: 10469890 Review.
Cited by
-
A Decades-Long Journey of Palmitoylethanolamide (PEA) for Chronic Neuropathic Pain Management: A Comprehensive Narrative Review.Pain Ther. 2025 Feb;14(1):81-101. doi: 10.1007/s40122-024-00685-4. Epub 2024 Dec 4. Pain Ther. 2025. PMID: 39630391 Free PMC article. Review.
-
An update on PPAR activation by cannabinoids.Br J Pharmacol. 2016 Jun;173(12):1899-910. doi: 10.1111/bph.13497. Epub 2016 May 19. Br J Pharmacol. 2016. PMID: 27077495 Free PMC article. Review.
-
Enhanced c-Fos expression in the central amygdala correlates with increased thigmotaxis in rats with peripheral nerve injury.Eur J Pain. 2016 Aug;20(7):1140-54. doi: 10.1002/ejp.839. Epub 2016 Mar 31. Eur J Pain. 2016. PMID: 27030378 Free PMC article.
-
Varicella zoster virus-induced pain and post-herpetic neuralgia in the human host and in rodent animal models.J Neurovirol. 2011 Dec;17(6):590-9. doi: 10.1007/s13365-011-0069-7. Epub 2011 Dec 28. J Neurovirol. 2011. PMID: 22205584 Free PMC article.
-
Serum levels of endocannabinoids and related lipids in painful vs painless diabetic neuropathy: results from the Pain in Neuropathy Study.Pain. 2024 Jan 1;165(1):225-232. doi: 10.1097/j.pain.0000000000003015. Epub 2023 Aug 11. Pain. 2024. PMID: 37578507 Free PMC article.
References
-
- Abdi S, Lee DH, Chung JM. The anti-allodynic effects of amitriptyline, gabapentin, and lidocaine in a rat model of neuropathic pain. Anesth Analg. 1998;87:1360–1366. - PubMed
-
- Ahmad KS, Rice ASC.Von Frey hairs compared with a force transducer for sensory measurement in normal and neuropathic rats 1999Poster Presentation at The Society for Neuroscience Annual Meeting Miami, FL
-
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, et al. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001;15:300–302. - PubMed
-
- Ben Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353:23–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

