Virodhamine and CP55,940 modulate cAMP production and IL-8 release in human bronchial epithelial cells
- PMID: 17558435
- PMCID: PMC2042924
- DOI: 10.1038/sj.bjp.0707320
Virodhamine and CP55,940 modulate cAMP production and IL-8 release in human bronchial epithelial cells
Abstract
Background and purpose: We investigated expression of cannabinoid receptors and the effects of the endogenous cannabinoid virodhamine and the synthetic agonist CP55,940 on cAMP accumulation and interleukin-8 (IL-8) release in human bronchial epithelial cells.
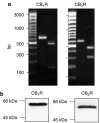
Experimental approach: Human bronchial epithelial (16HBE14o(-)) cells were used. Total mRNA was isolated and cannabinoid receptor mRNAs were detected by RT-PCR. Expression of CB(1) and CB(2) receptor proteins was detected with Western blotting using receptor-specific antibodies. cAMP accumulation was measured by competitive radioligand binding assay. IL-8 release was measured by ELISA.
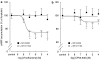
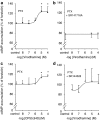
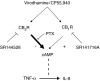
Key results: CB(1) and CB(2) receptor mRNAs and proteins were found. Both agonists concentration-dependently decreased forskolin-induced cAMP accumulation. This effect was inhibited by the CB(2) receptor antagonist SR144528, and was sensitive to Pertussis toxin (PTX), suggesting the involvement of CB(2) receptors and G(i/o)-proteins. Cell pretreatment with PTX unmasked a stimulatory component, which was blocked by the CB(1) receptor antagonist SR141716A. CB(2) receptor-mediated inhibition of cAMP production by virodhamine and CP55,940 was paralleled by inhibition of tumor necrosis factor-alpha (TNF-alpha) induced IL-8 release. This inhibition was insensitive to SR141716A. In the absence of agonist, SR144528 by itself reduced TNF-alpha induced IL-8 release.
Conclusions and implications: Our results show for the first time that 16HBE14o(-) cells respond to virodhamine and CP55,940. CB(1) and CB(2) receptor subtypes mediated activation and inhibition of adenylyl cyclase, respectively. Stimulation of the dominant CB(2) receptor signalling pathway diminished cAMP accumulation and TNF-alpha-induced IL-8 release. These observations may imply that cannabinoids exert anti-inflammatory properties in airways by modulating cytokine release.
Figures



Similar articles
-
(Endo)cannabinoids mediate different Ca2+ entry mechanisms in human bronchial epithelial cells.Naunyn Schmiedebergs Arch Pharmacol. 2009 Jul;380(1):67-77. doi: 10.1007/s00210-009-0406-z. Epub 2009 Mar 3. Naunyn Schmiedebergs Arch Pharmacol. 2009. PMID: 19255745
-
A synthetic cannabinoid, CP55940, inhibits lipopolysaccharide-induced cytokine mRNA expression in a cannabinoid receptor-independent mechanism in rat cerebellar granule cells.J Pharm Pharmacol. 2011 May;63(5):636-47. doi: 10.1111/j.2042-7158.2011.01250.x. Epub 2011 Mar 28. J Pharm Pharmacol. 2011. PMID: 21492165
-
Cannabinoid CB1 receptor-mediated modulation of evoked dopamine release and of adenylyl cyclase activity in the human neocortex.Br J Pharmacol. 2004 Apr;141(7):1193-203. doi: 10.1038/sj.bjp.0705706. Epub 2004 Mar 1. Br J Pharmacol. 2004. PMID: 14993102 Free PMC article.
-
Inhibition of the cAMP signaling cascade via cannabinoid receptors: a putative mechanism of immune modulation by cannabinoid compounds.Toxicol Lett. 1998 Dec 28;102-103:59-63. doi: 10.1016/s0378-4274(98)00284-7. Toxicol Lett. 1998. PMID: 10022233 Review.
-
CB(1) cannabinoid receptors and their associated proteins.Curr Med Chem. 2010;17(14):1382-93. doi: 10.2174/092986710790980023. Curr Med Chem. 2010. PMID: 20166926 Free PMC article. Review.
Cited by
-
Disease modification of breast cancer-induced bone remodeling by cannabinoid 2 receptor agonists.J Bone Miner Res. 2013 Jan;28(1):92-107. doi: 10.1002/jbmr.1732. J Bone Miner Res. 2013. PMID: 22903605 Free PMC article.
-
(Endo)cannabinoids mediate different Ca2+ entry mechanisms in human bronchial epithelial cells.Naunyn Schmiedebergs Arch Pharmacol. 2009 Jul;380(1):67-77. doi: 10.1007/s00210-009-0406-z. Epub 2009 Mar 3. Naunyn Schmiedebergs Arch Pharmacol. 2009. PMID: 19255745
-
HDAC 3-selective inhibitor RGFP966 demonstrates anti-inflammatory properties in RAW 264.7 macrophages and mouse precision-cut lung slices by attenuating NF-κB p65 transcriptional activity.Biochem Pharmacol. 2016 May 15;108:58-74. doi: 10.1016/j.bcp.2016.03.010. Epub 2016 Mar 16. Biochem Pharmacol. 2016. PMID: 26993378 Free PMC article.
-
ACPA decreases non-small cell lung cancer line growth through Akt/PI3K and JNK pathways in vitro.Cell Death Dis. 2021 Jan 11;12(1):56. doi: 10.1038/s41419-020-03274-3. Cell Death Dis. 2021. PMID: 33431819 Free PMC article. Clinical Trial.
-
The CB2 receptor and its role as a regulator of inflammation.Cell Mol Life Sci. 2016 Dec;73(23):4449-4470. doi: 10.1007/s00018-016-2300-4. Epub 2016 Jul 11. Cell Mol Life Sci. 2016. PMID: 27402121 Free PMC article. Review.
References
-
- Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998;50:515–596. - PubMed
-
- Berdyshev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 2000;108:169–190. - PubMed
-
- Bonhaus DW, Chang LK, Kwan J, Martin GR. Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther. 1998;287:884–888. - PubMed
-
- Burkey TH, Quock RM, Consroe P, Ehlert FJ, Hosohata Y, Roeske WR, et al. Relative efficacies of cannabinoid CB1 receptor agonists in the mouse brain. Eur J Pharmacol. 1997;336:295–298. - PubMed
-
- Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

