Generation and characterization of an ascitogenic mesothelin-expressing tumor model
- PMID: 17559144
- PMCID: PMC3181493
- DOI: 10.1002/cncr.22781
Generation and characterization of an ascitogenic mesothelin-expressing tumor model
Abstract
Background: Intraperitoneal tumors expressing high amounts of mesothelin such as malignant mesothelioma and ovarian cancers tend to develop ascites and result in significant morbidity and mortality in the patient. A suitable preclinical intraperitoneal model will assist in the illustration of the mechanisms of molecular oncogenesis and facilitate in addressing issues related to early screening, diagnosis, and therapy for intraperitoneal tumors.
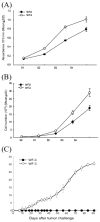
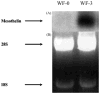
Methods: In the current study, an ascitogenic malignant tumor model (WF-3) was created. The mobility and proliferation of WF-3 and its precursor cells, WF-0, were characterized using transwell and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assays. In addition, the in vivo tumorgenicity of WF-3 and WF-0 was determined using intraperitoneal injection of the tumor cells. Microarray analysis was performed using WF-3 and WF-0. Northern blot analysis was used to characterize the expression of the mesothelin gene in WF-3 and WF-0. Furthermore, the mesothelin levels in serum and ascites were used to correlate with tumor load of WF-3 in tumor challenged mice.
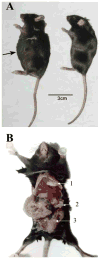
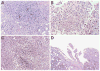
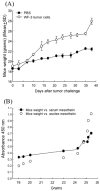
Results: The WF-3 tumor cells demonstrated relatively high proliferation and migration rates compared with the parental cell line, WF-0. The tumors from the WF-3 but not WF-0 were capable of forming ascites and peritoneal-based tumors after tumor challenge. The WF-3 tumor model was also capable of implanting into multiple organs including the diaphragm, intestines, and peritoneal wall. Furthermore, the WF-3 tumor expressed high levels of mesothelin, which is commonly observed in the majority of ovarian cancers, pancreatic cancer, and malignant mesothelioma. In addition, the authors found that the serum and ascites mesothelin levels correlated with tumor loads in tumor-challenged mice.
Conclusions: The data indicate that the WF-3 murine tumor model may potentially serve as a good model for understanding the molecular oncogenesis of peritoneal tumors. In addition, the preclinical model may potentially be useful for the development of diagnostic and therapeutic methods against intraperitoneal cancers.
Figures




References
-
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. - PubMed
-
- Schink JC. Current initial therapy of stage III and IV ovarian cancer: challenges for managed care. Semin Oncol. 1999;26:2–7. - PubMed
-
- Zellos LS, Sugarbaker DJ. Multimodality treatment of diffuse malignant pleural mesothelioma. Semin Oncol. 2002;29:41–50. - PubMed
-
- Nowak AK, Lake RA, Kindler HL, Robinson BW. New approaches for mesothelioma: biologics, vaccines, gene therapy, and other novel agents. Semin Oncol. 2002;29:82–96. - PubMed
-
- Liu J, Yang G, Thompson-Lanza JA, et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–1663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

