Novel rat Alzheimer's disease models based on AAV-mediated gene transfer to selectively increase hippocampal Abeta levels
- PMID: 17559680
- PMCID: PMC1906777
- DOI: 10.1186/1750-1326-2-11
Novel rat Alzheimer's disease models based on AAV-mediated gene transfer to selectively increase hippocampal Abeta levels
Abstract
Background: Alzheimer's disease (AD) is characterized by a decline in cognitive function and accumulation of amyloid-beta peptide (Abeta) in extracellular plaques. Mutations in amyloid precursor protein (APP) and presenilins alter APP metabolism resulting in accumulation of Abeta42, a peptide essential for the formation of amyloid deposits and proposed to initiate the cascade leading to AD. However, the role of Abeta40, the more prevalent Abeta peptide secreted by cells and a major component of cerebral Abeta deposits, is less clear. In this study, virally-mediated gene transfer was used to selectively increase hippocampal levels of human Abeta42 and Abeta40 in adult Wistar rats, allowing examination of the contribution of each to the cognitive deficits and pathology seen in AD.
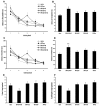
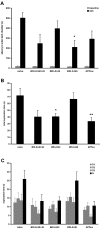
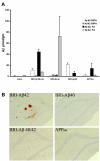
Results: Adeno-associated viral (AAV) vectors encoding BRI-Abeta cDNAs were generated resulting in high-level hippocampal expression and secretion of the specific encoded Abeta peptide. As a comparison the effect of AAV-mediated overexpression of APPsw was also examined. Animals were tested for development of learning and memory deficits (open field, Morris water maze, passive avoidance, novel object recognition) three months after infusion of AAV. A range of impairments was found, with the most pronounced deficits observed in animals co-injected with both AAV-BRI-Abeta40 and AAV-BRI-Abeta42. Brain tissue was analyzed by ELISA and immunohistochemistry to quantify levels of detergent soluble and insoluble Abeta peptides. BRI-Abeta42 and the combination of BRI-Abeta40+42 overexpression resulted in elevated levels of detergent-insoluble Abeta. No significant increase in detergent-insoluble Abeta was seen in the rats expressing APPsw or BRI-Abeta40. No pathological features were noted in any rats, except the AAV-BRI-Abeta42 rats which showed focal, amorphous, Thioflavin-negative Abeta42 deposits.
Conclusion: The results show that AAV-mediated gene transfer is a valuable tool to model aspects of AD pathology in vivo, and demonstrate that whilst expression of Abeta42 alone is sufficient to initiate Abeta deposition, both Abeta40 and Abeta42 may contribute to cognitive deficits.
Figures


Similar articles
-
Amyloid β peptides overexpression in retinal pigment epithelial cells via AAV-mediated gene transfer mimics AMD-like pathology in mice.Sci Rep. 2017 Jun 12;7(1):3222. doi: 10.1038/s41598-017-03397-2. Sci Rep. 2017. PMID: 28607377 Free PMC article.
-
Comparison of neurotoxicity of different aggregated forms of Aβ40, Aβ42 and Aβ43 in cell cultures.J Pept Sci. 2017 Mar;23(3):245-251. doi: 10.1002/psc.2975. Epub 2017 Feb 16. J Pept Sci. 2017. PMID: 28211253
-
Calcium-sensing receptor antagonist (calcilytic) NPS 2143 specifically blocks the increased secretion of endogenous Aβ42 prompted by exogenous fibrillary or soluble Aβ25-35 in human cortical astrocytes and neurons-therapeutic relevance to Alzheimer's disease.Biochim Biophys Acta. 2013 Oct;1832(10):1634-52. doi: 10.1016/j.bbadis.2013.04.020. Epub 2013 Apr 26. Biochim Biophys Acta. 2013. PMID: 23628734
-
[The beta-amyloid cascade hypothesis: a sequence of events leading to neurodegeneration in Alzheimer's disease].Neurol Neurochir Pol. 2004 Sep-Oct;38(5):405-11. Neurol Neurochir Pol. 2004. PMID: 15565529 Review. Polish.
-
Neurodevelopmental disorders and microcephaly: how apoptosis, the cell cycle, tau and amyloid-β precursor protein APPly.Front Mol Neurosci. 2023 Sep 22;16:1201723. doi: 10.3389/fnmol.2023.1201723. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37808474 Free PMC article. Review.
Cited by
-
Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia.Nat Neurosci. 2009 Oct;12(10):1300-7. doi: 10.1038/nn.2395. Epub 2009 Sep 6. Nat Neurosci. 2009. PMID: 19734892
-
Secreted Chaperones in Neurodegeneration.Front Aging Neurosci. 2020 Aug 27;12:268. doi: 10.3389/fnagi.2020.00268. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33192447 Free PMC article. Review.
-
Adeno-Associated Viruses for Modeling Neurological Diseases in Animals: Achievements and Prospects.Biomedicines. 2022 May 15;10(5):1140. doi: 10.3390/biomedicines10051140. Biomedicines. 2022. PMID: 35625877 Free PMC article. Review.
-
Gene Therapy Models of Alzheimer's Disease and Other Dementias.Methods Mol Biol. 2016;1382:339-66. doi: 10.1007/978-1-4939-3271-9_25. Methods Mol Biol. 2016. PMID: 26611599 Free PMC article.
-
Alzheimer's disease: experimental models and reality.Acta Neuropathol. 2017 Feb;133(2):155-175. doi: 10.1007/s00401-016-1662-x. Epub 2016 Dec 26. Acta Neuropathol. 2017. PMID: 28025715 Free PMC article. Review.
References
-
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. - DOI - PMC - PubMed
-
- Herzig MC, Winkler DT, Burgermeister P, Pfeifer M, Kohler E, Schmidt SD, Danner S, Abramowski D, Sturchler-Pierrat C, Burki K, van Duinen SG, Maat-Schieman ML, Staufenbiel M, Mathews PM, Jucker M. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci. 2004;7:954–960. doi: 10.1038/nn1302. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

