alphaCaMKII autophosphorylation levels differ depending on subcellular localization
- PMID: 17559813
- PMCID: PMC2077298
- DOI: 10.1016/j.brainres.2007.05.008
alphaCaMKII autophosphorylation levels differ depending on subcellular localization
Abstract
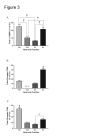
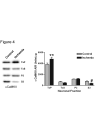
Calcium/calmodulin-dependent protein kinase II (CaMKII) has important roles in many processes in the central nervous system. It is enriched at the post-synaptic density (PSD), a localization which is thought to be critical for many of its proposed neuronal functions. In order to better understand the mechanisms that regulate association of CaMKII with the PSD, we compared the levels of autophosphorylation between PSD-associated kinase and kinase in other parts of the neuron. We were surprised to find that alphaCaMKII in a PSD-enriched fraction prepared from recovered hippocampal CA1-minislices had a relatively low level of threonine 286 (T286) phosphorylation and a relatively high level of threonine 305 (T305) phosphorylation. Furthermore, when the minislices were subjected to a treatment that mimics ischemic conditions, there was a significant translocation of alphaCaMKII to the PSD-enriched fraction accompanied with a dramatic reduction in T286 phosphorylation levels throughout the neuron. These findings have important implications for our understanding of the role of autophosphorylation in the localization of CaMKII.
Figures



Similar articles
-
CaMKII-dependent phosphorylation of NR2A and NR2B is decreased in animals characterized by hippocampal damage and impaired LTP.Eur J Neurosci. 1999 Jan;11(1):141-8. doi: 10.1046/j.1460-9568.1999.00414.x. Eur J Neurosci. 1999. PMID: 9987018
-
Hippocampal synaptic plasticity involves competition between Ca2+/calmodulin-dependent protein kinase II and postsynaptic density 95 for binding to the NR2A subunit of the NMDA receptor.J Neurosci. 2001 Mar 1;21(5):1501-9. doi: 10.1523/JNEUROSCI.21-05-01501.2001. J Neurosci. 2001. PMID: 11222640 Free PMC article.
-
A structural mechanism for maintaining the 'on-state' of the CaMKII memory switch in the post-synaptic density.J Neurochem. 2007 Oct;103(1):357-64. doi: 10.1111/j.1471-4159.2007.04744.x. J Neurochem. 2007. PMID: 17877639 Free PMC article.
-
Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function.Annu Rev Biochem. 2002;71:473-510. doi: 10.1146/annurev.biochem.71.110601.135410. Epub 2001 Nov 9. Annu Rev Biochem. 2002. PMID: 12045104 Review.
-
Activity-dependent regulation of calcium/calmodulin-dependent protein kinase II localization.J Neurosci. 2004 Sep 29;24(39):8399-403. doi: 10.1523/JNEUROSCI.3606-04.2004. J Neurosci. 2004. PMID: 15456811 Free PMC article. Review. No abstract available.
Cited by
-
Excitotoxic insult results in a long-lasting activation of CaMKIIα and mitochondrial damage in living hippocampal neurons.PLoS One. 2015 Mar 20;10(3):e0120881. doi: 10.1371/journal.pone.0120881. eCollection 2015. PLoS One. 2015. PMID: 25793533 Free PMC article.
-
Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca²⁺-permeable AMPA receptors during the incubation of cocaine craving.Neuropharmacology. 2011 Dec;61(7):1141-51. doi: 10.1016/j.neuropharm.2011.01.021. Epub 2011 Jan 27. Neuropharmacology. 2011. PMID: 21276808 Free PMC article.
-
Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking.Neuropharmacology. 2012 Jun;62(7):2430-9. doi: 10.1016/j.neuropharm.2012.02.017. Epub 2012 Feb 22. Neuropharmacology. 2012. PMID: 22387532 Free PMC article.
-
Arc ubiquitination regulates endoplasmic reticulum-mediated Ca2+ release and CaMKII signaling.Front Cell Neurosci. 2023 Mar 14;17:1091324. doi: 10.3389/fncel.2023.1091324. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36998269 Free PMC article.
-
Rapid and sustained GluA1 S845 phosphorylation in synaptic and extrasynaptic locations in the rat forebrain following amphetamine administration.Neurochem Int. 2014 Jan;64:48-54. doi: 10.1016/j.neuint.2013.11.001. Epub 2013 Nov 11. Neurochem Int. 2014. PMID: 24231469 Free PMC article.
References
-
- Aronowski J, Grotta JC, Waxham MN. Ischemia-induced translocation of Ca2+/calmodulin-dependent protein kinase II: potential role in neuronal damage. J Neurochem. 1992;58:1743–1753. - PubMed
-
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. - PubMed
-
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. - PubMed
-
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

