Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex
- PMID: 17559940
- PMCID: PMC2171036
- DOI: 10.1016/j.brainresrev.2007.05.002
Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex
Abstract
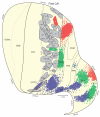
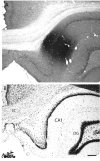
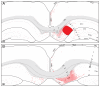
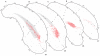
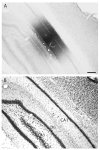
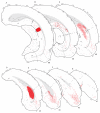
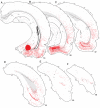
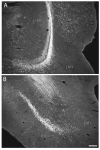
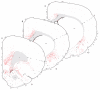
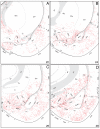
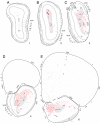
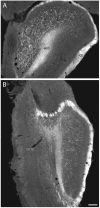
The spatial distribution of axonal projections descending from rat field CA1 to thalamus and hypothalamus was analyzed previously with the PHAL method [Cenquizca, L.A., Swanson, L.W. 2006. An analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol 497:101-114.]. The same experimental material was used here to define the topography of field CA1 association projections to other cerebral cortical areas. First, the results confirm and extend known intrahippocampal formation inputs to dentate gyrus, subiculum, presubiculum, parasubiculum, and entorhinal area, which are arranged generally along the formation's transverse axis and dominated by the subicular projection-by far the densest established by field CA1 anywhere in the brain. And second, field CA1 innervates a virtually complete ring of extrahippocampal formation cortex via three routes. A dorsal pathway from the dorsal third of field CA1 innervates moderately the retrosplenial area; a moderately strong ventral pathway from the ventral two thirds of field CA1 passing through the longitudinal association bundle sends offshoots to visual, auditory, somatosensory, gustatory, main and accessory olfactory, and visceral areas-as well as the basolateral amygdalar complex and the agranular insular and orbital areas; and a cortical-subcortical-cortical pathway through the fornix from the whole longitudinal extent of field CA1 innervates rather strongly a rostral region that includes the tenia tecta along with the anterior cingulate, prelimbic, infralimbic, and orbital areas. The functional consequences of long-term potentiation in field CA1 projection neurons remain to be explored.
Figures






References
-
- Acsady L, Arabadzisz D, Freund TF. Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neurosci. 1996;73:299–315. - PubMed
-
- Acsady L, Pascual M, Rocamora N, Soriano E, Freund TF. Nerve growth factor but not neurotrophin-3 is synthesized by hippocampal GABAergic neurons that project to the medial septum. Neurosci. 2000;98:23–31. - PubMed
-
- Adams CE, Stitzel JA, Collins AC, Freedman R. Alpha7-nicotinic receptor expression and the anatomical organization of hippocampal interneurons. Brain Res. 2001;922:180–190. - PubMed
-
- Altemus KL, Lavenex P, Ishizuka N, Amaral DG. Morphological characteristics and electrophysiological properties of CA1 pyramidal neurons in macaque monkeys. Neurosci. 2005;136:741–756. - PubMed
-
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. Academic Press; San Diego: 1995. pp. 443–493.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

