Integrin-mediated laminin-1 adhesion upregulates CXCR4 and IL-8 expression in pancreatic cancer cells
- PMID: 17560257
- PMCID: PMC1994963
- DOI: 10.1016/j.surg.2006.12.016
Integrin-mediated laminin-1 adhesion upregulates CXCR4 and IL-8 expression in pancreatic cancer cells
Abstract
Background: We have shown recently that alpha(2)beta(1) integrin-mediated type I collagen adhesion promotes a more malignant phenotype in pancreatic cancer cell lines than other extracellular matrix (ECM) proteins. MiaPaCa-2 cells, by contrast, do not express collagen-binding integrins, but are metastatic in our orthotopic mouse model and migrate maximally on laminin-1 (Ln-1). It has also been shown that CXCR4 and IL-8 expression correlates directly with metastasis in pancreatic cancer in vivo. We therefore examined the potential of the ECM to regulate CXCR4 and IL-8 expression in pancreatic cancer cells.
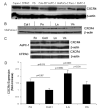
Methods: We cultured 8 pancreatic cancer cell lines on fibronectin (Fn), types I and IV collagen, Ln-1 and vitronectin (Vn), and examined cell lysates for CXCR4 by immunoblotting and media for IL-8 by ELISA. We also conducted cell migration assays with stromal-derived factor-1 (SDF-1) as the chemoattractant to examine integrin-binding specificity and CXCR4 function.
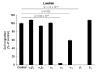
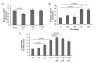
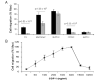
Results: All cell lines expressed CXCR4 protein. MiaPaCa-2 cell growth on Ln-1 increased significantly CXCR4 and IL-8 expression relative to other ECM proteins. Migration inhibition studies showed that both the alpha(6)beta(1) and alpha(3)beta(1) integrins mediate MiaPaCa-2 migration on Ln-1. Growth studies showed further that CXCR4 expression on Ln-1 was mediated by the alpha(6)beta(1) integrin whereas IL-8 expression was mediated by both the alpha(6)beta(1) and alpha(3)beta(1) integrins. The expression of functional CXCR4 was also shown in migration assays, where SDF-1 significantly increased pancreatic cancer cell chemotaxis on Ln-1.
Conclusions: These data indicate that integrin-mediated Ln-1 adhesion upregulates CXCR4 and IL-8 expression and may play a mechanistic role in pancreatic cancer metastases.
Figures

References
-
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. - PubMed
-
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. - PubMed
-
- Weinel RJ, Rosendahl A, Neumann K, Chaloupka B, Erb D, Rothmund M, et al. Expression and function of VLA-alpha 2, -alpha 3, -alpha 5 and -alpha 6-integrin receptors in pancreatic carcinoma. Int J Cancer. 1992;52:827–33. - PubMed
-
- Rosendahl A, Neumann K, Chaloupka B, Rothmund M, Weinel RJ. Expression and distribution of VLA receptors in the pancreas: an immunohistochemical study. Pancreas. 1993;8:711–8. - PubMed
-
- Shimoyama S, Gansauge F, Gansauge S, Oohara T, Beger HG. Altered expression of extracellular matrix molecules and their receptors in chronic pancreatitis and pancreatic adenocarcinoma in comparison with normal pancreas. Int J Pancreatol. 1995;18:227–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

