An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction
- PMID: 17560295
- PMCID: PMC2857596
- DOI: 10.1016/j.jacc.2007.02.050
An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction
Abstract
Objectives: Our objective in this study was to apply an elastic, biodegradable polyester urethane urea (PEUU) cardiac patch onto subacute infarcts and to examine the resulting cardiac ventricular remodeling and performance.
Background: Myocardial infarction induces loss of contractile mass and scar formation resulting in adverse left ventricular (LV) remodeling and subsequent severe dysfunction.
Methods: Lewis rats underwent proximal left coronary ligation. Two weeks after coronary ligation, a 6-mm diameter microporous PEUU patch was implanted directly on the infarcted LV wall surface (PEUU patch group, n = 14). Sham surgery was performed as an infarction control (n = 12). The LV contractile function, regional myocardial wall compliance, and tissue histology were assessed 8 weeks after patch implantation.
Results: The end-diastolic LV cavity area (EDA) did not change, and the fractional area change (FAC) increased in the PEUU patch group (p < 0.05 vs. week 0), while EDA increased and FAC decreased in the infarction control group (p < 0.05). The PEUU patch was largely resorbed 8 weeks after implantation and the LV wall was thicker than infarction control (p < 0.05 vs. control group). Abundant smooth muscle bundles with mature contractile phenotype were found in the infarcted myocardium of the PEUU group. The myocardial compliance of the PEUU group was distributed between normal myocardium and infarction control (p < 0.001).
Conclusions: Implantation of a novel biodegradable PEUU patch onto a subacute myocardial infarction promoted contractile phenotype smooth muscle tissue formation and improved cardiac remodeling and contractile function at the chronic stage. Our findings suggest a new therapeutic option against post-infarct cardiac failure.
Figures




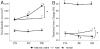
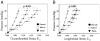
References
-
- Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–49. - PubMed
-
- Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–53. - PubMed
-
- White HD, Norris RM, Brown MA, Brandt PW, Whitlock PM, Wild CJ. Left ventricular end-systolic volume as the major predictor of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. - PubMed
-
- Sabbah HN, Shimoyama H, Kono T, et al. Effects of long-term monotherapy with enalapril, metoprolol, and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation. 1994;89:2852–9. - PubMed
-
- Dor V, Sabatier M, DiDonato M, Montiglio F, Toso A, Maioli M. Efficacy of endoventricular patch plasty in large postinfarction akinetic scar and severe left ventricular dysfunction: comparison with a series of large dyskinetic scars. J Thorac Cardiovasc Surg. 1998;116:50–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

