Inhibition of the HERG potassium channel by the tricyclic antidepressant doxepin
- PMID: 17560554
- PMCID: PMC1920586
- DOI: 10.1016/j.bcp.2007.04.024
Inhibition of the HERG potassium channel by the tricyclic antidepressant doxepin
Abstract
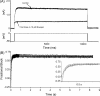
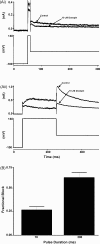
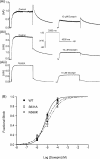
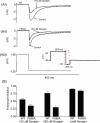
HERG (human ether-à-go-go-related gene) encodes channels responsible for the cardiac rapid delayed rectifier potassium current, I(Kr). This study investigated the effects on HERG channels of doxepin, a tricyclic antidepressant linked to QT interval prolongation and cardiac arrhythmia. Whole-cell patch-clamp recordings were made at 37 degrees C of recombinant HERG channel current (I(HERG)), and of native I(Kr) 'tails' from rabbit ventricular myocytes. Doxepin inhibited I(HERG) with an IC(50) value of 6.5+/-1.4 microM and native I(Kr) with an IC(50) of 4.4+/-0.6 microM. The inhibitory effect on I(HERG) developed rapidly upon membrane depolarization, but with no significant dependence on voltage and with little alteration to the voltage-dependent kinetics of I(HERG). Neither the S631A nor N588K inactivation-attenuating mutations (of residues located in the channel pore and external S5-Pore linker, respectively) significantly reduced the potency of inhibition. The S6 point mutation Y652A increased the IC(50) for I(HERG) blockade by approximately 4.2-fold; the F656A mutant also attenuated doxepin's action at some concentrations. HERG channel blockade is likely to underpin reported cases of QT interval prolongation with doxepin. Notably, this study also establishes doxepin as an effective inhibitor of mutant (N588K) HERG channels responsible for variant 1 of the short QT syndrome.
Figures




References
-
- Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999;354:1625–1633. - PubMed
-
- Haverkamp W., Breithardt G., Camm A.J., Janse M.J., Rosen M.R., Antzelevitch C. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications report on a policy conference of the European Society of Cardiology. Cardiovasc Res. 2000;47:219–233. - PubMed
-
- Shah R.R. Drugs QT interval prolongation and ICH E14—the need to get it right. Drug Safety. 2005;28:115–125. - PubMed
-
- Witchel H.J., Hancox J.C. Familial and acquired long QT syndrome and the cardiac rapid delayed rectifier potassium current. Clin Exp Pharmacol Physiol. 2000;27:753–766. - PubMed
-
- Vandenberg J.I., Walker B.D., Campbell T.J. HERG K+ channels: friend and foe. Trends Pharmacol Sci. 2001;22:240–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

