A novel loop domain in superantigens extends their T cell receptor recognition site
- PMID: 17560605
- PMCID: PMC2949350
- DOI: 10.1016/j.jmb.2007.05.038
A novel loop domain in superantigens extends their T cell receptor recognition site
Abstract
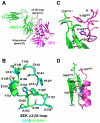
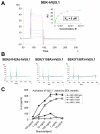
Superantigens (SAGs) interact with host immune receptors to induce a massive release of inflammatory cytokines that can lead to toxic shock syndrome and death. Bacterial SAGs can be classified into five distinct evolutionary groups. Group V SAGs are characterized by the alpha3-beta8 loop, a unique approximately 15 amino acid residue extension that is required for optimal T cell activation. Here, we report the X-ray crystal structures of the group V SAG staphylococcal enterotoxin K (SEK) alone and in complex with the TCR hVbeta5.1 domain. SEK adopts a unique TCR binding orientation relative to other SAG-TCR complexes, which results in the alpha3-beta8 loop contacting the apical loop of framework region 4, thereby extending the known TCR recognition site of SAGs. These interactions are absolutely required for TCR binding and T cell activation by SEK, and dictate the TCR Vbeta domain specificity of SEK and other group V SAGs.
Figures



References
-
- McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. - PubMed
-
- Sundberg EJ, Li Y, Mariuzza RA. So many ways of getting in the way: diversity in the molecular architecture of superantigen-dependent T-cell signaling complexes. Curr Opin Immunol. 2002;14:36–44. - PubMed
-
- Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Schwartz B, O'Rourke K, Talbot J, Low DE. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome--a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis. 1999;28:800–7. - PubMed
-
- Li Y, Li H, Dimasi N, McCormick JK, Martin R, Schuck P, Schlievert PM, Mariuzza RA. Crystal structure of a superantigen bound to the high-affinity, zinc-dependent site on MHC class II. Immunity. 2001;14:93–104. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

