Abnormal motor phenotype in the SMNDelta7 mouse model of spinal muscular atrophy
- PMID: 17561409
- PMCID: PMC2700002
- DOI: 10.1016/j.nbd.2007.04.009
Abnormal motor phenotype in the SMNDelta7 mouse model of spinal muscular atrophy
Abstract
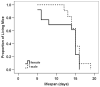
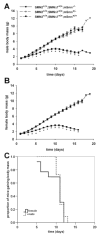
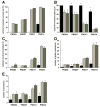
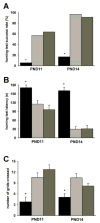
Spinal muscular atrophy (SMA) is a recessive motor neuron disease that affects motor neurons in the anterior horn of the spinal cord. SMA results from the reduction of SMN (survival motor neuron) protein. Even though SMN is ubiquitously expressed, motor neurons are more sensitive to the reduction in SMN than other cell types. We have previously generated mouse models of SMA with varying degrees of clinical severity. So as to more clearly understand the pathogenesis of motor neuron degeneration in SMA, we have characterized the phenotype of the SMNDelta7 SMA mouse which normally lives for 13.6+/-0.7 days. These mice are smaller than their non-SMA littermates and begin to lose body mass at 10.4+/-0.4 days. SMNDelta7 SMA mice exhibit impaired responses to surface righting, negative geotaxis and cliff aversion but not to tactile stimulation. Spontaneous motor activity and grip strength are also significantly impaired in SMNDelta7 SMA mice. In summary, we have demonstrated an impairment of neonatal motor responses in SMNDelta7 SMA mice. This phenotype characterization could be used to assess the effectiveness of potential therapies for SMA.
Figures



References
-
- Altafaj X, Dierssen M, Baamonde C, Martí E, Visa J, Guimerà J, Oset M, González JR, Flórez J, Fillat C, Estivill X. Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A minibrain), a murine model of Down’s syndrome. Hum Mol Genet. 2001;10:1915–1923. - PubMed
-
- Amendola J, Verrier B, Roubertoux P, Durand J. Altered sensorimotor development in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2004;20:2822–2826. - PubMed
-
- Azzouz M, Leclerc N, Gurney M, Warter JM, Poindron P, Borg J. Progressive motor neuron impairment in an animal model of familial amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:45–51. - PubMed
-
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. - PubMed
-
- Butchbach MER, Burghes AHM. Perspectives on models of spinal muscular atrophy for drug discovery. Drug Discov Today Dis Model. 2004;1:151–156.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

