Interleukin 25 promotes the initiation of proallergic type 2 responses
- PMID: 17562814
- PMCID: PMC2118650
- DOI: 10.1084/jem.20061675
Interleukin 25 promotes the initiation of proallergic type 2 responses
Abstract
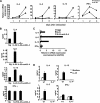
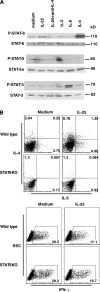
The molecular mechanisms underlying the initiation of innate and adaptive proallergic type 2 responses are not understood. Interleukin (IL) 25, a member of the IL-17 cytokine family, was recently reported (Owyang, A.M., C. Zaph, E.H. Wilson, K.J. Guild, T. McClanahan, H.R. Miller, D.J. Cua, M. Goldschmidt, C.A. Hunter, R.A. Kastelein, and D. Artis. 2006. J. Exp. Med. 203:843-849; Fallon, P.G., S.J. Ballantyne, N.E. Mangan, J.L. Barlow, A. Dasvarma, D.R. Hewett, A. McIlgorm, H.E. Jolin, and A.N. McKenzie. 2006. J. Exp. Med. 203:1105-1116) to be important in Th2 cell-mediated immunity to parasitic infection. However, the cellular source and targets of IL-25 are not well understood. We show that mouse IL-25 is expressed by lung epithelial cells as a result of innate immune responses to allergens. Transgenic overexpression of IL-25 by these cells leads to mucus production and airway infiltration of macrophages and eosinophils, whereas blockade of IL-25 conversely reduces the airway inflammation and Th2 cytokine production in an allergen-induced asthma model. In addition, IL-25, with a receptor more highly expressed in Th2 than other effector T cells, promotes Th2 cell differentiation in an IL-4- and signal transducer and activator of transcription 6-dependent manner. During early T cell activation, IL-25 potentiates expression of the nuclear factor of activated T cells c1 and JunB transcription factors, which possibly results in increased levels of initial IL-4 production, up-regulation of GATA-3 expression, and enhanced Th2 cell differentiation. Thus, IL-25 is a critical factor regulating the initiation of innate and adaptive proallergic responses.
Figures



References
-
- Murphy, K.M. 1998. T lymphocyte differentiation in the periphery. Curr. Opin. Immunol. 10:226–232. - PubMed
-
- Dong, C. 2006. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 6:329–334. - PubMed
-
- Dong, C., and R.A. Flavell. 2000. Control of T helper cell differentiation—in search of master genes. Sci. STKE. 2000:PE1. - PubMed
-
- Hurst, S.D., T. Muchamuel, D.M. Gorman, J.M. Gilbert, T. Clifford, S. Kwan, S. Menon, B. Seymour, C. Jackson, T.T. Kung, et al. 2002. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 169:443–453. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

